Session Information
The 2020 Pediatric Rheumatology Symposium, originally scheduled for April 29 – May 2, was postponed due to COVID-19; therefore, abstracts were not presented as scheduled.
Date: Friday, May 1, 2020
Title: Poster Session 2
Session Type: ACR Abstract Session
Session Time: 5:00PM-6:00PM
Background/Purpose: Testing for ANCA, particularly performed by ELISA (anti-MPO/PR3) is highly sensitive and specific for ANCA-associated vasculitis (AAV). However ANCA testing may be used in clinical scenarios with low likelihoods for AAV, leading to a decreased positive predictive value. While non-AAV diagnoses associated with positive testing for ANCA in adult populations have been reported, clinical scenarios in children leading to testing for ANCA testing in children are likely different. This study characterized the diagnoses and outcomes associated with positive tests for ANCA in children.
Methods: Retrospective cohort study of all testing of ANCA performed in pediatric patients (≤18 years old) in a single healthcare system (mixed academic and non-academic) representing over 2/3 of the healthcare in the state from 2004-2017. Chart data was extracted on patients who were positive on their first test for ANCA by IIF or ELISA. Patient positive for ANCA were divided into three groups: 1. AAV; 2. Autoimmune gastrointestinal disease (inflammatory bowel disease (IBD) Crohn’s disease, Ulcerative colitis (UC), undifferentiated IBD, autoimmune hepatitis (AIH), and primary sclerosing cholangitis (PSC); 3. Other diagnoses/No diagnosis. Follow-up time was defined as time from initial ANCA testing to time of last available visit.
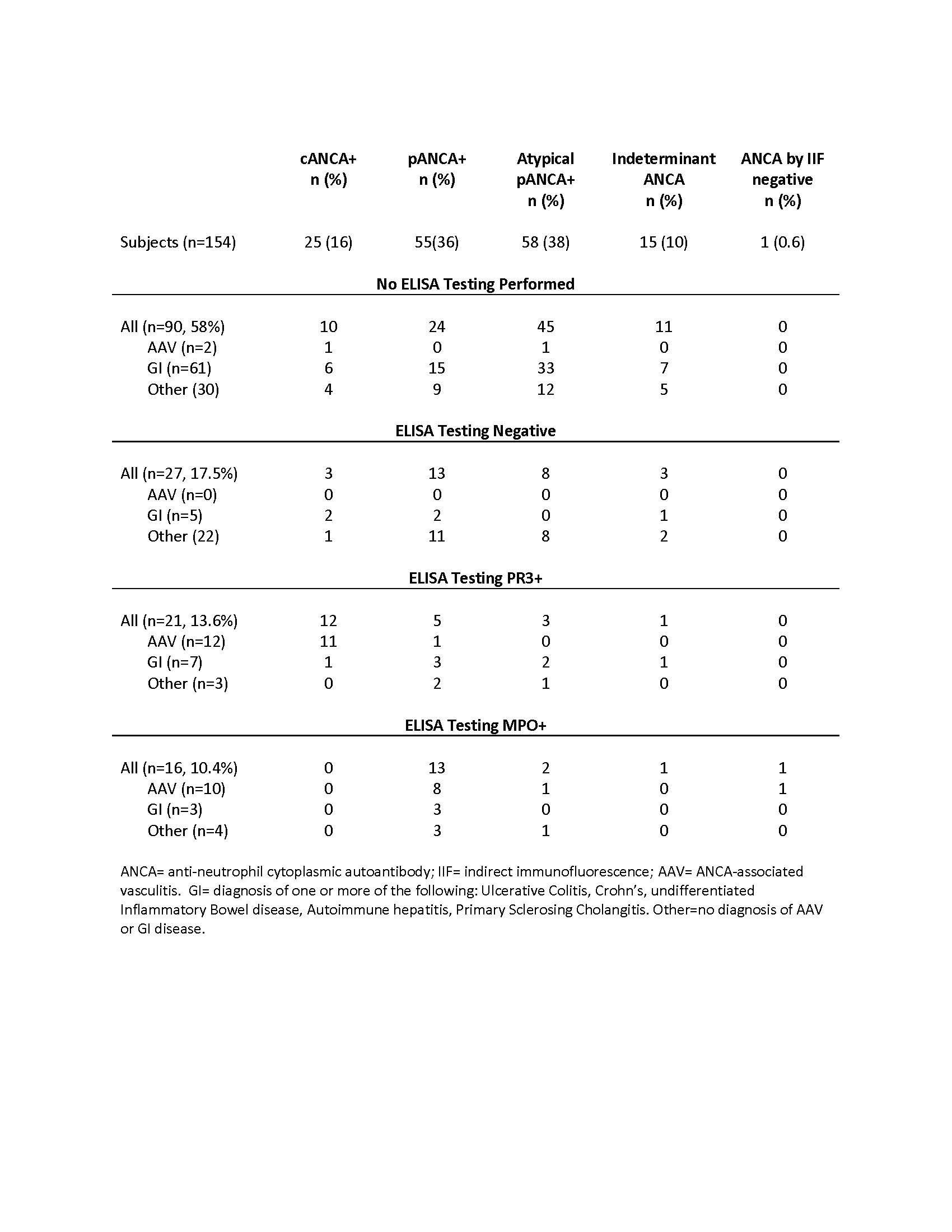
Results: Testing for ANCA was performed on 2,931 patients, of whom 154 (5.3%) were positive for ANCA by IIF and/or ELISA. There was an increase in testing of ANCA from 2004 to 2016 (p=0.027). Of 154 subjects positive for ANCA, all were tested by IIF and 64 (41%) were tested by ELISA. 153/154 (99%) were positive by IIF and 37/64 (58%) were positive by ELISA. 24 (16%) patients received a diagnosis of AAV, 1 was diagnosed with UC < 1 year from time of her diagnosis of AAV (pANCA/PR3+), and 1 had a preceding diagnosis of AIH (pANCA/MPO+). 62 (40%) received a diagnosis of IBD, 19 of whom had concurrent AIH or PSC, and 14 with AIH/PSC without IBD. 56 (36%) of patients positive for ANCA did not have a diagnosis of AAV or autoimmune GI disease. ANCA type and diagnosis category are shown in Table 1. 7/76 (9%) of patients with autoimmune GI disease had a positive ANCA by ELISA. 20/33 (61%) GI and 14/30 (47%) with other diagnoses who were c or pANCA positive had a titer of > 1:80. The diagnoses of the patients without AAV or GI disease are shown in Table 2.
All but 2 patients with AAV were diagnosed within 6 weeks of the initial testing for ANCA; 2 were diagnosed later, after seeking second opinion. No other patients who were positive for ANCA and not initially diagnosed with AAV (n=130) subsequently developed AAV, with a median of 4.7 years of follow up (IQR 2.4, 10.3).
Conclusion: The majority of positive tests for ANCA occurring in children are associated with diseases other than AAV, especially IBD, AIH, and PSC. Positive tests for ANCA occur in a variety of autoimmune and infectious diseases in children, even at moderate to high titers. The clinical importance of a positive test for ANCA in children without AAV is not clear; however, a positive test for ANCA in the absence of concurrent evidence of AAV does not appear to confer risk for later development of AAV.
To cite this abstract in AMA style:
James K, Merkel P, Hersh A. Implications of Positive Tests for ANCA in a Pediatric Population [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 4). https://acrabstracts.org/abstract/implications-of-positive-tests-for-anca-in-a-pediatric-population/. Accessed .« Back to 2020 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/implications-of-positive-tests-for-anca-in-a-pediatric-population/