Session Information
Date: Sunday, November 8, 2020
Title: Pediatric Rheumatology – Clinical Poster II: Systemic JIA, Autoinflammatory, & Scleroderma
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Macrophage activation syndrome (MAS) and hemophagocytic lymphohistiocytosis (HLH) epitomize a diverse and deadly group of inflammatory hyperferritinemic syndromes. Early biomarkers distinguishing these syndromes, especially those indicating specific treatments, are lacking.
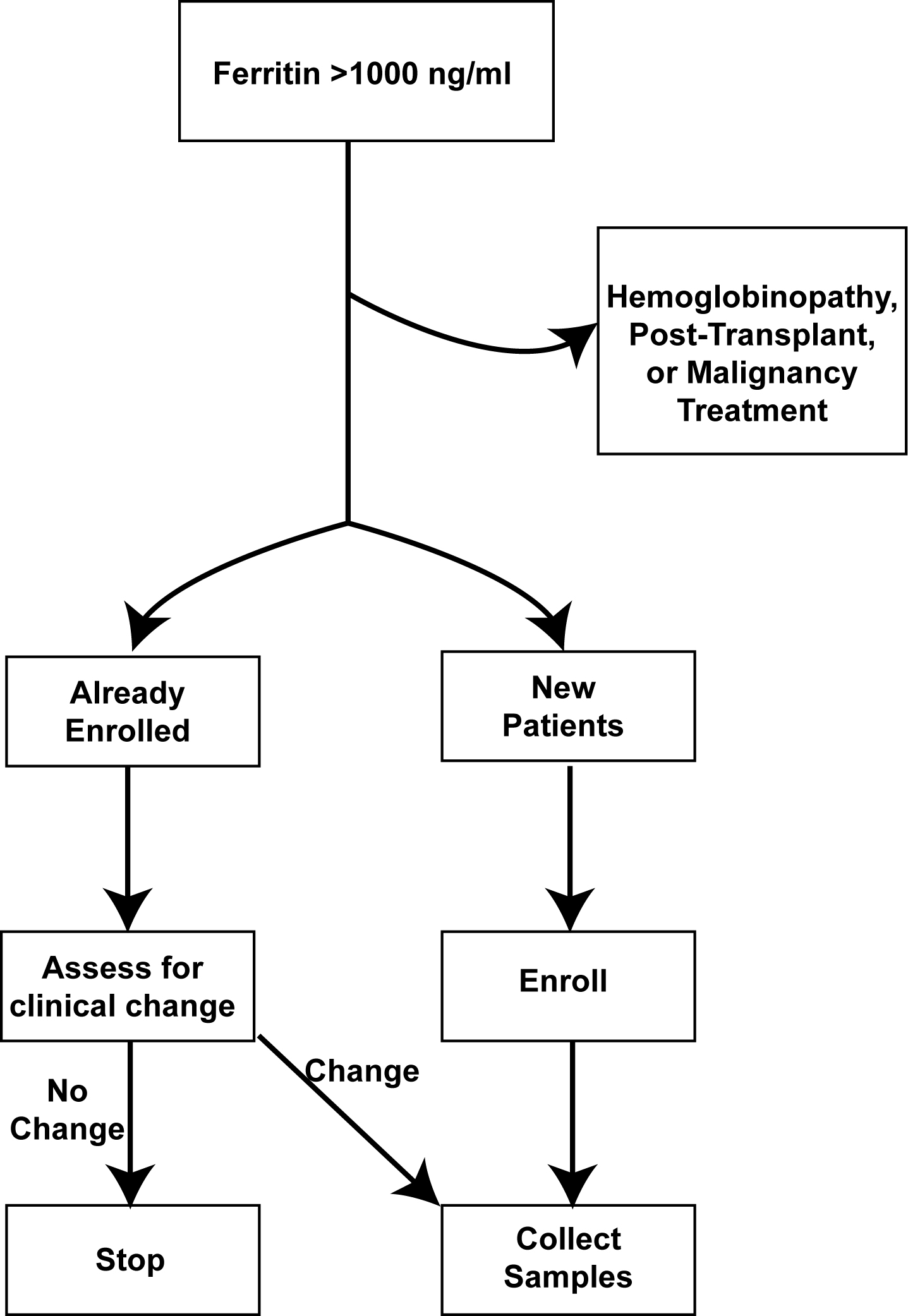
Methods: .Between 6/2017 to 6/2019, we instituted a hyperferritinemic alert system for serum ferritin >1000ng/mL (Figure 1). Positive alerts were screened via real-time chart review and categorized into inflammatory hyperferritinemia, hemoglobinopathy (e.g. sickle cell disease), malignancy, post-transplant (solid organ and hematopoietic), and miscellaneous. The earliest available leftover serum samples from inflammatory hyperferritinemia alerts were set aside by and frozen in our clinical laboratory. Such patients were contacted for enrollment into an ongoing natural history protocol, and samples from patients able to be (or previously) enrolled were collected and tested. Longitudinal samples were obtained when possible focusing on changes in clinical status. Subsequently, we divided the inflammatory hyperferritinemia patients into infectious, rheumatic (all of whom were systemic JIA, or SJIA, during this period), or immune dysregulation. We extracted laboratory elements of MAS/HLH from enrolled patients’ electronic medical record (AST, CRP, platelets) and measured IL-18, IL-18 binding protein (IL-18BP), and CXCL9 on stored samples. All procedures were approved by the University of Pittsburgh Institutional Review Board (STUDY20010099).
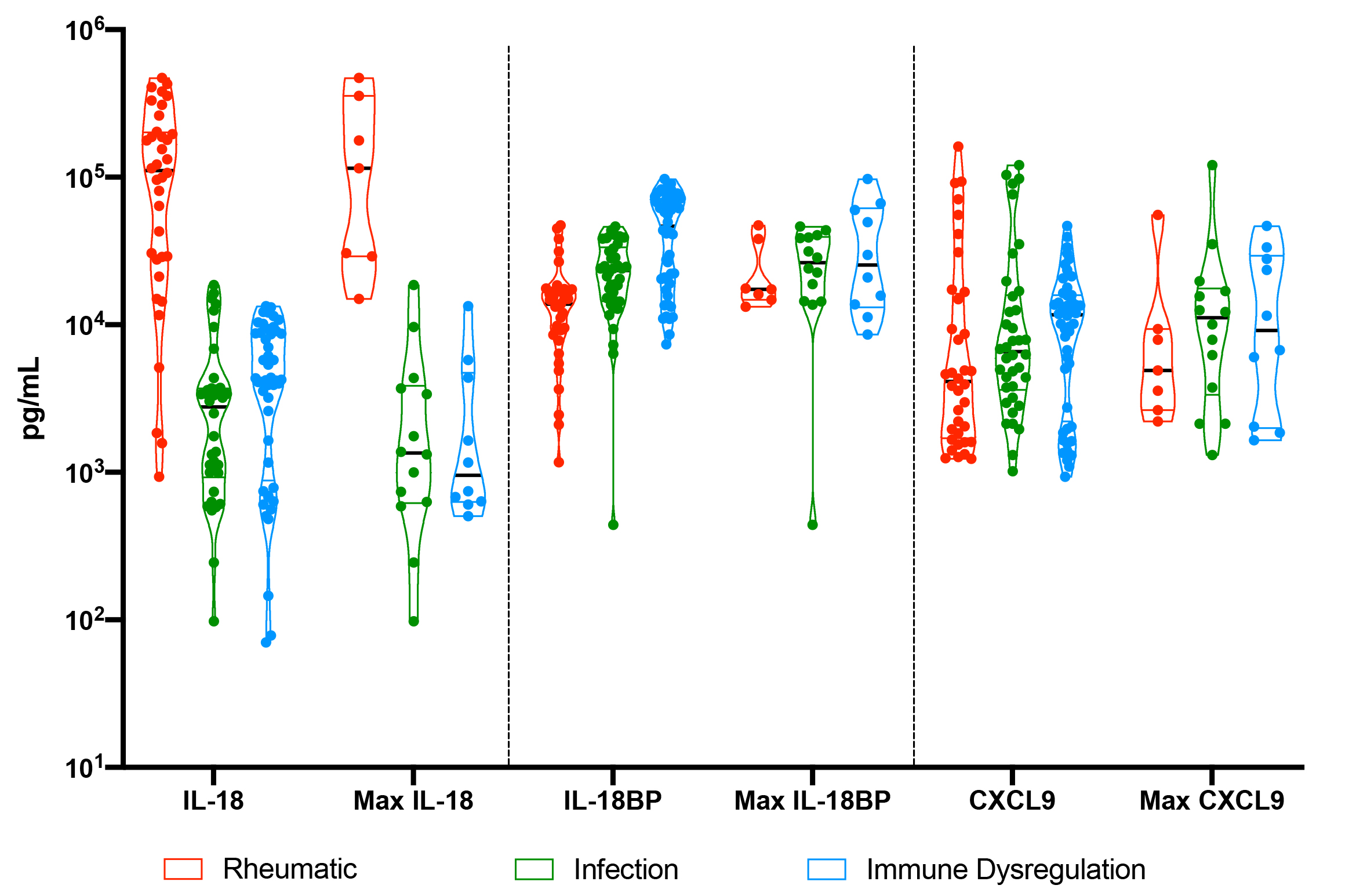
Results: We screened 931 alerts from 180 unique patients. Inflammatory hyperferritinemia was adjudicated as the cause of 40.3% of screened ferritin levels from 30.5% of distinct patients. Maximum ferritin levels were higher in patients with inflammatory hyperferritinemia compared to hemoglobinopathies, malignancies, post-transplant, and miscellaneous diseases. Among the inflammatory hyperferritinemia group, the highest AST levels were observed in patients with “immune dysregulation”, whereas maximum CRP levels were higher in infectious and SJIA-associated hyperinflammatory states. Platelets were less-consistently depressed in SJIA-associated hyperferritinemia. Consistent with previous findings, highly elevated IL-18 was uniquely observed in samples from patients with SJIA/MAS, whereas this group showed slightly less IL-18BP and CXCL9 elevation (Figure 2). Longitudinal analysis showed that IL-18 correlated with active or “smoldering” MAS activity (Figure 3).
Conclusion: Prospective screening based on hyperferritinemia is a feasible way to identify patients and collect early samples. Extremely elevated serum IL-18 was consistently observed in patients with SJIA, and improved pathophysiologic understanding in patients with unclear diagnoses (e.g. Figure 3). This protocol would be amenable to lower triggering thresholds and expanded biomarker screening to further optimize identification, diagnosis, and treatment of MAS/HLH and related diseases.
To cite this abstract in AMA style:
Zhang M, Schneider C, Dang V, Canna S. Implementation and Initial Experience with a Screening Protocol for Inflammatory Hyperferritinemia [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/implementation-and-initial-experience-with-a-screening-protocol-for-inflammatory-hyperferritinemia/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/implementation-and-initial-experience-with-a-screening-protocol-for-inflammatory-hyperferritinemia/