Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
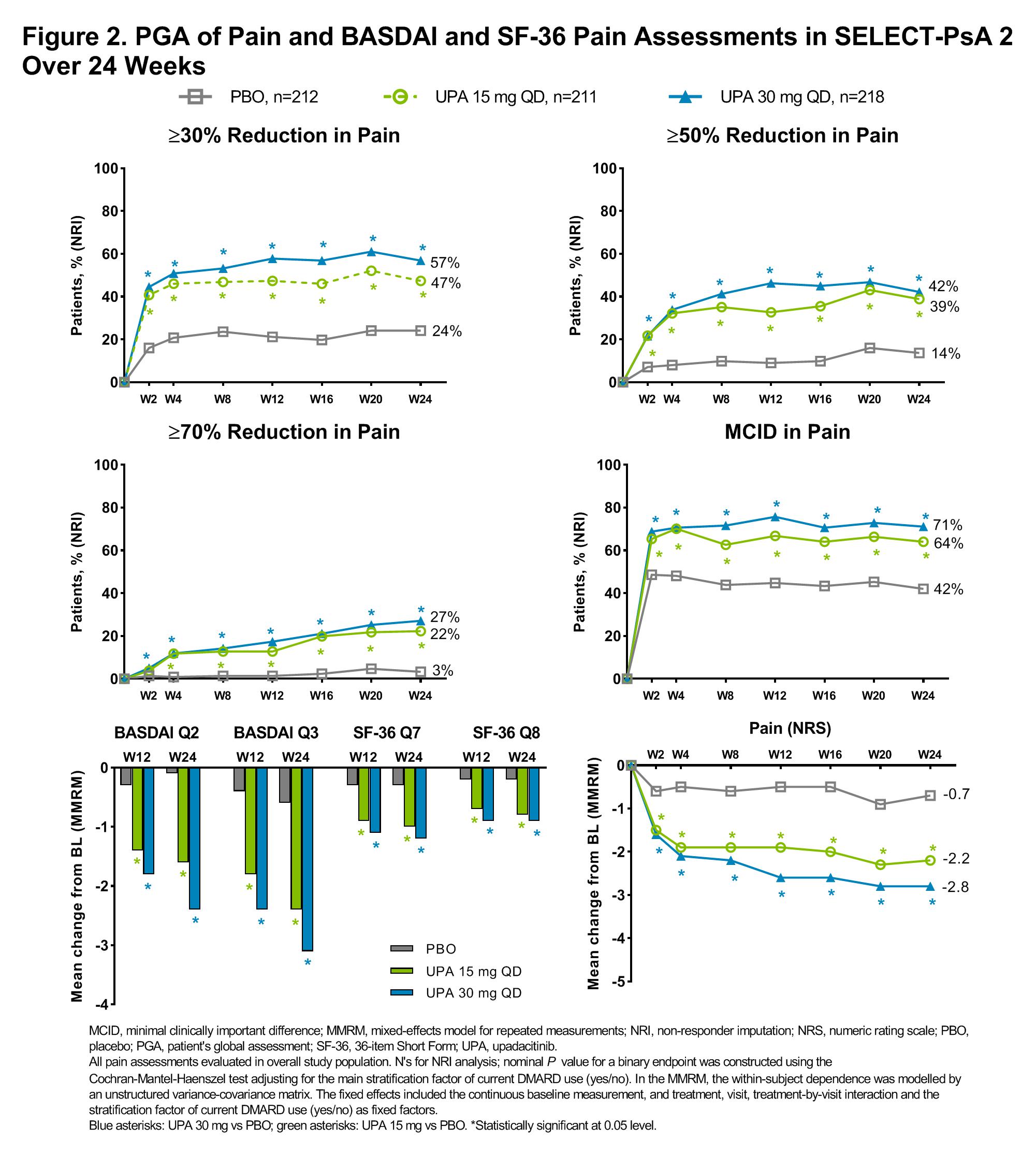
Background/Purpose: Pain is a dominant symptom of psoriatic arthritis (PsA), and pain reduction is a priority for patients (pts) that is often assessed in clinical trials. Upadacitinib (UPA), a Janus kinase (JAK) inhibitor engineered for increased selectivity for JAK1 over JAK2, JAK3, and tyrosine kinase2, has demonstrated safety and efficacy in pts with active PsA in the SELECT-PsA 1 and 2 studies.1,2 The objective of this analysis was to compare the efficacy of UPA vs placebo (PBO) and adalimumab (ADA) on pain using different assessments through 24 weeks (wks).
Methods: The SELECT-PsA program enrolled adult pts with active PsA with prior inadequate response (IR) or intolerance to ≥1 non-biologic DMARD (SELECT-PsA 1; NCT03104400) or prior IR or intolerance to ≥1 biologic DMARD (SELECT-PsA 2; NCT03104374). Concomitant background therapy with ≤2 non-biologic DMARDs was allowed but not required. Pts were randomized to UPA 15 mg or UPA 30 mg once daily (QD) or PBO (both studies), or ADA 40 mg every other week (EOW; SELECT-PsA 1 only). Pain was assessed as proportion of pts achieving ≥30%, ≥50%, or ≥70% reduction from baseline (BL) in Pt’s global assessment (PGA) of pain numeric rating scale (NRS) score (0–10), proportion of pts achieving minimal clinically important difference (MCID) in pain (defined as ≥1 point reduction or 15% reduction from BL on a 0–10 NRS)3,4 and change from baseline in pain NRS (0–10) at all time points. In addition, change from BL in BASDAI questions 2 (spinal pain) and 3 (joint pain/swelling) and 36-Item Short Form Survey (SF-36) questions 7 (bodily pain) and 8 (pain interference) at weeks 12 and 24 were assessed. Non-responder imputation was used for binary endpoints and mixed-effects model for repeated measurements for continuous endpoints. The statistical significance defined as P< 0.05 was exploratory in nature.
Results: In both studies, a significantly higher proportion of pts receiving UPA 15 mg QD and UPA 30 mg QD vs PBO achieved improvements in most pain endpoints as early as wk 2, and improvements were generally either sustained or increased through wk 24 (nominal P<0.05; Figure 1 and 2). A significant improvement with UPA vs PBO was also observed for change from BL in PGA of pain NRS scores over time, as well as in BASDAI spinal pain and joint pain/swelling and SF-36 bodily pain and pain interference at weeks 12 and 24 (Figure 1 and 2). In SELECT-PsA 1 significantly higher proportions of pts receiving UPA 30 mg QD vs ADA 40 mg EOW achieved improvements in most pain assessments as early as wk 2 which were sustained through wk 24; improvements in several assessments were also significantly greater with UPA 15 mg QD vs ADA 40 mg EOW at wk 24 (nominal P < 0.05; Figure 1).
Conclusion: In pts with active PsA who had inadequate response to non-biologic or biologic DMARDs, a greater proportion of pts treated with UPA vs PBO achieved rapid, significant, and clinically meaningful reductions in pain across multiple pain assessments. The reductions in pain were sustained over 24 wks.
- McInnes I. et al. Ann Rheum Dis. 2020;79(Suppl 1):12-13.
- Genovese M.C. et al. Ann Rheum Dis. 2020;79(Suppl 1):139.
- Dworkin, R.H. et al. J Pain. 2008;9(2):105-121.
- Salaffi F. et al. Eur J Pain. 2004;8:283–291.
To cite this abstract in AMA style:
McInnes I, Tillett W, Mease P, de Vlam K, Bessette L, Lippe R, Maniccia A, Zueger P, Feng D, Kato K, Östör A. Impact of Upadacitinib on Reducing Pain in Patients with Active Psoriatic Arthritis: Results from Two Phase 3 Trials in Patients with Inadequate Response to Non-biologic or Biologic DMARDs [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/impact-of-upadacitinib-on-reducing-pain-in-patients-with-active-psoriatic-arthritis-results-from-two-phase-3-trials-in-patients-with-inadequate-response-to-non-biologic-or-biologic-dmards/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-upadacitinib-on-reducing-pain-in-patients-with-active-psoriatic-arthritis-results-from-two-phase-3-trials-in-patients-with-inadequate-response-to-non-biologic-or-biologic-dmards/