Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Ankylosing spondylitis (AS) patients (pts) were found to respond better to TNF inhibitors (TNFi) if treated early in the disease course. The objective of this analysis was to examine the impact of time since diagnosis, age, and number of prior NSAIDs as surrogates for disease duration on clinical outcomes in AS pts treated with adalimumab (ADA) in ATLAS trial.
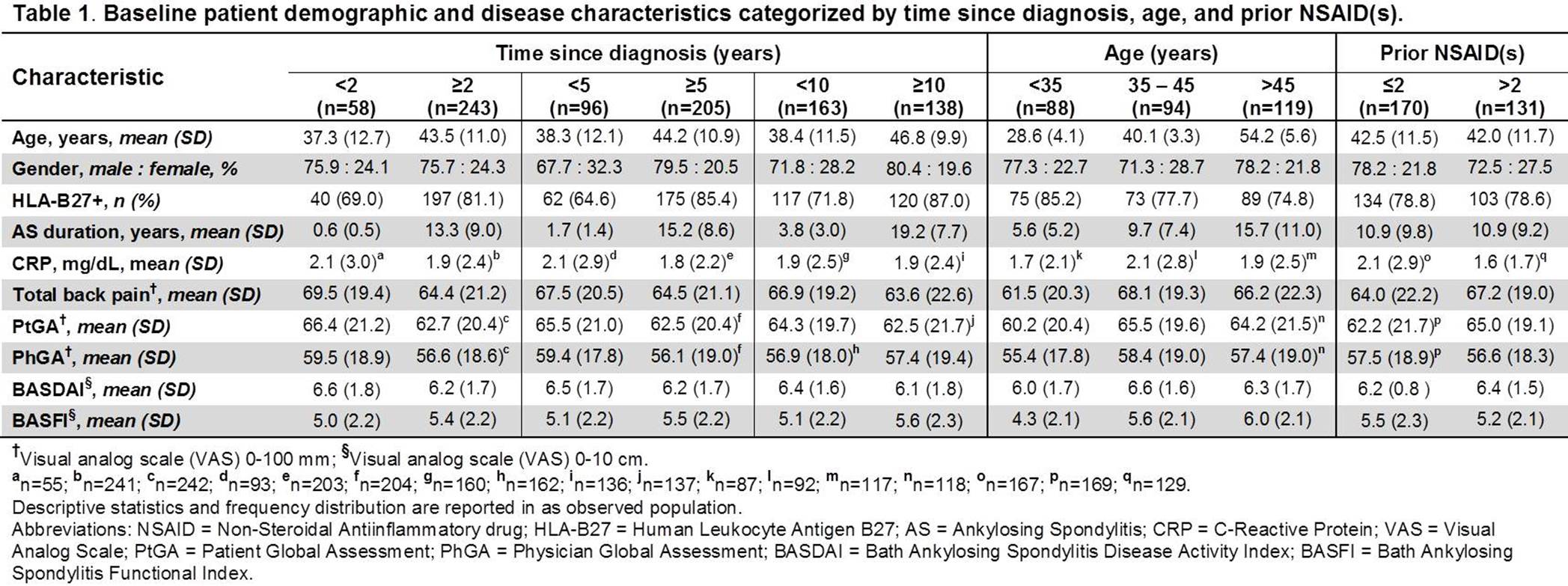
Methods: ATLAS1,2 was a phase III randomized double-blind trial comparing safety and efficacy of ADA with placebo (PBO) in pts with active AS who failed NSAID therapy. In this post hoc analysis, pts were categorized by baseline (BL) (1) time since diagnosis (<2 vs ≥2, <5 vs ≥5, and <10 vs ≥10 years [yrs]), (2) age (<35, 35 – 45, and >45 yrs), and (3) number of prior NSAIDs (≤ 2 vs >2). The effect of time since diagnosis, age and number of prior NSAIDs on AS outcome measures at week (wk) 12 was determined in pts who received at least one dose of ADA during the PBO-controlled period or open label extension and received prior NSAID(s) at BL.
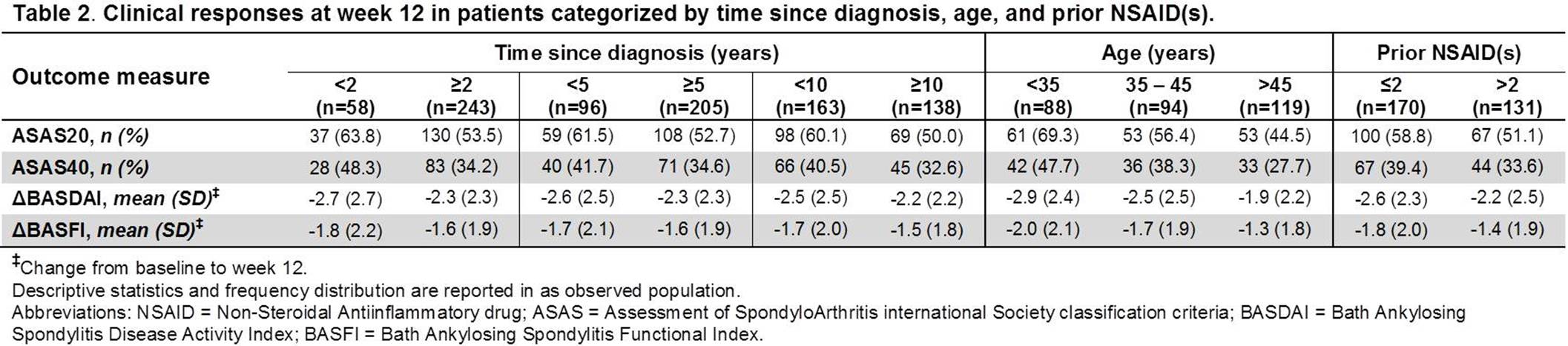
Results: Of the 301 pts included in this analysis, a majority of them had ≥5 yrs since AS diagnosis (205 [68.1%]), were <45 yrs of age (182 [60.5%]), and had ≤2 prior NSAIDs (170 [56.5%]). Pts with shorter time since diagnosis were generally younger, while those with longer time since diagnosis were more frequently HLA-B27+. There were more men than women across all subcategories. CRP was numerically higher in patients with ≤2 prior NSAIDs. The BL disease activity measures were however numerically similar across most categories (Table 1). At wk 12, the proportions of pts achieving ASAS20 and ASAS40 responses were numerically higher and mean decreases in BASDAI and BASFI scores from baseline larger in subcategories with shorter time since diagnosis, lower age, and fewer prior NSAIDs (Table 2). The differences in the clinical outcomes were significant between <35 and >45 yrs age categories (ASAS20: P<0.001; ASAS40: P=0.003; BASDAI: P=0.002; BASFI: P=0.012).
Conclusion: Shorter time since diagnosis, younger age, and lower number of prior NSAIDs resulted in numerically greater clinical responses and improvements in disease activity and functionality following ADA treatment in pts with AS. The younger age (<35 yrs) had the largest positive impact on the clinical outcomes as compared with higher age (>45 yrs), suggesting its closest association with the actual disease duration. Overall, the results support the need for early effective treatment intervention in AS pts to improve clinical outcomes. References:
- Van der Heijde, D. et al., Ann Rheum Dis, 2007; 67:1218-21.
- Sieper, J. et al., Ann Rheum Dis, 2012; 71:700-6.
To cite this abstract in AMA style:
Sieper J, Deodhar AA, Hojnik M, Zhang Y, Dougados M. Impact of Time Since Diagnosis, Age, and Number of Prior Non-Steroidal Anti-Inflammatory Drugs on Response to Adalimumab (HUMIRA) in Patients with Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/impact-of-time-since-diagnosis-age-and-number-of-prior-non-steroidal-anti-inflammatory-drugs-on-response-to-adalimumab-humira-in-patients-with-ankylosing-spondylitis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-time-since-diagnosis-age-and-number-of-prior-non-steroidal-anti-inflammatory-drugs-on-response-to-adalimumab-humira-in-patients-with-ankylosing-spondylitis/