Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The Covid-19 pandemic constituted major challenges for health-care services worldwide. We aimed to compare Covid-19 restrictions across Europe during the first three waves of the pandemic, as well as the consultation and follow-up practices of patients with spondyloarthritis before and during the pandemic.
Methods: Rheumatologists completed a Research Electronic Data Capture (REDCap) survey in 13 observational registries in the European Spondyloarthritis Research Collaboration Network (EuroSpA) between July 1st and October 27,th 2022: ATTRA (Czech Republic), DANBIO (Denmark), ESRBTR (Estonia), ROB-FIN (Finland), ICEBIO (Iceland), GISEA (Italy), ARC (Netherlands), NOR-DMARD (Norway), Reuma.pt (Portugal), RRBR (Romania), biorx.si (Slovenia), BIOBADASER (Spain), and SCQM (Switzerland), answering questions on Covid-19 restrictions in their individual countries and the impact of Covid-19 on consultation and follow-up of routine care patients with spondyloarthritis in their registries. The survey covered the period from the start of the pandemic in the different countries, until 1st of March, 2022.
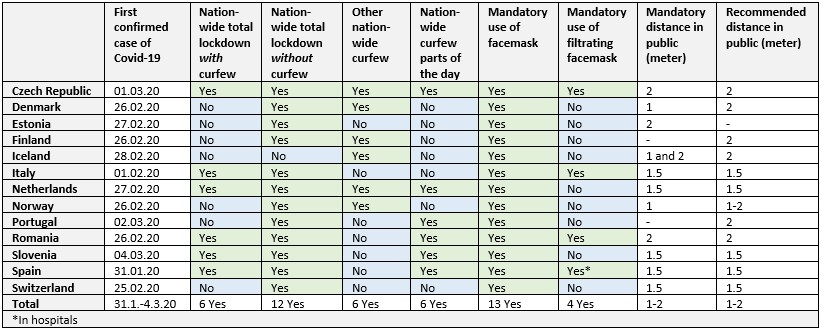
Results: The first case of Covid-19 in each country was reported between 31st of January 2020 in Spain and 4th of March 2020 in Slovenia. Use of facemask in public was mandatory in all countries during the peak of the pandemic, but use of filtering facemask (type P2 or P3) only in four countries (Table 1). All countries except for Iceland had a nationwide total lockdown. Most countries had nationwide curfews, which occasionally applied to specific periods throughout the day. All countries had social distancing varying between one and two meters from others in public. This was mandatory at some time-point during the pandemic in all countries, except in Finland and Portugal, where it was recommended.
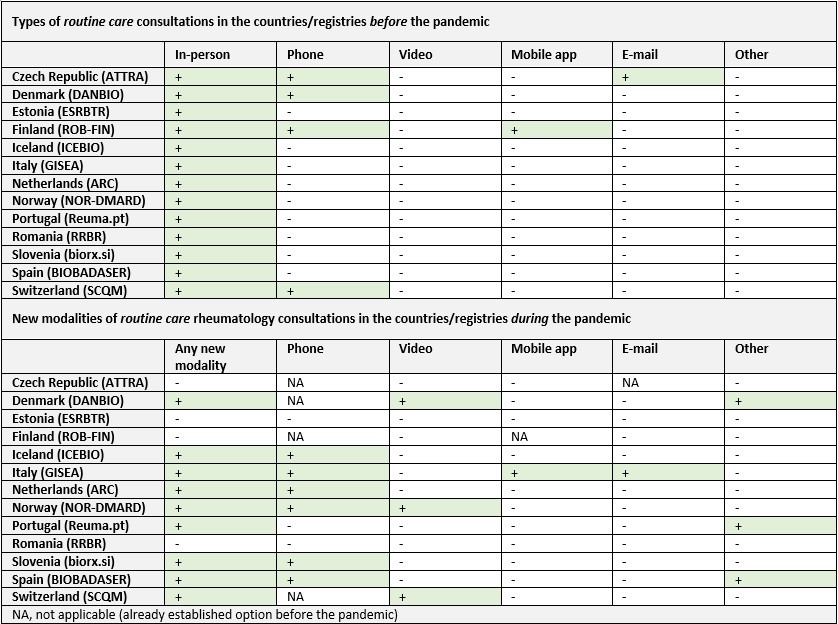
Before the pandemic, only four countries/registries had alternative modes of consultation in addition to the usual routine care physical consultations (Table 2). However, during the pandemic, nine registries initiated other modes of routine care consultations, the most common being phone consultations. The new modalities of consultations were not mandatory in any registry, except for mandatory non-urgent remote consultations in Switzerland during the first wave of the pandemic (phone or video, Table 3). In addition, in Spain, phone consultations and consultations between primary care physicians and rheumatologists were mandatory at some hospitals.
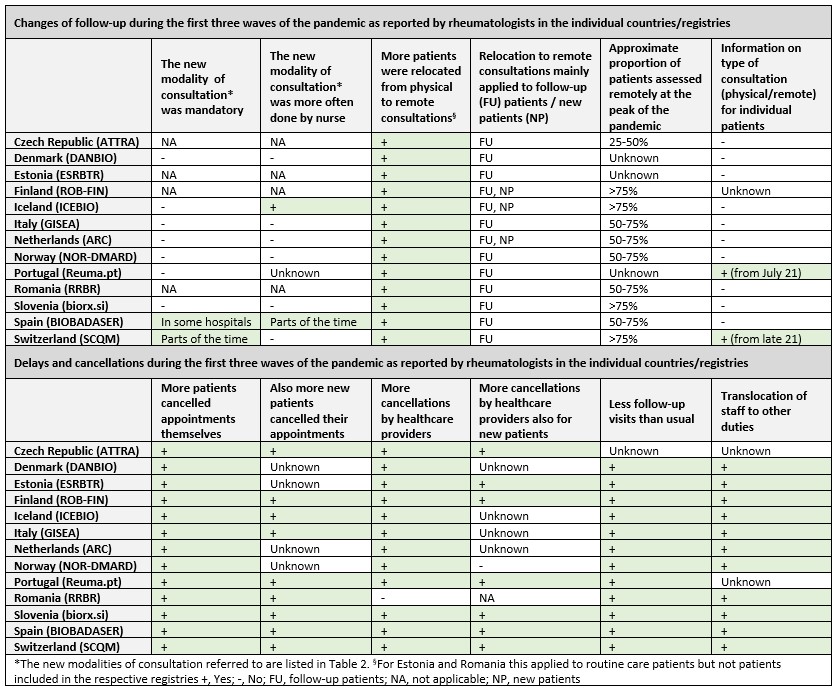
In every registry, rheumatologists reported an increased patient relocation from physical to remote consultations during the pandemic. At the peak of the pandemic, most of the patients were assessed remotely; however, this mainly applied to follow-up patients. More patients and healthcare providers canceled appointments during the pandemic than before. Furthermore, in all registries, staff was translocated to other duties and patients had fewer follow-up consultations than usual.
Conclusion: The Covid-19 pandemic had far-reaching consequences across Europe, not only due to nationwide restrictions, but also as a result of its negative impact on the accessibility of rheumatological care and follow-up.
To cite this abstract in AMA style:
Michelsen B, Glintborg B, Lauper K, Gudbjornsson B, Ørnbjerg L, Gröndal G, Laas K, Vorobjov S, Nordstrom D, Relas H, Ciurea A, Moeller B, Castrejon I, Otero-Valera L, Rotar Z, Perdan Pirkmajer K, Loft A, Zavada J, Pavelka K, Kristianslund E, Kvien T, van de Sande M, Hellamand P, Iannone F, Caporali R, Rodrigues A, Santos M, Codreanu C, Mogosan C, Hetland M, Østergaard M. Impact of the First Three Waves of the Covid-19 Pandemic on Everyday Restrictions and Clinical Care of Patients with Spondyloarthritis Across Europe – Results from the EuroSpA Collaboration [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/impact-of-the-first-three-waves-of-the-covid-19-pandemic-on-everyday-restrictions-and-clinical-care-of-patients-with-spondyloarthritis-across-europe-results-from-the-eurospa-collaboration/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-the-first-three-waves-of-the-covid-19-pandemic-on-everyday-restrictions-and-clinical-care-of-patients-with-spondyloarthritis-across-europe-results-from-the-eurospa-collaboration/