Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: In general, renal elimination is minimal for therapeutic proteins with a molecular weight more than 69 KDa. However, in patients with proteinuria, there is impaired filtration or insufficiency of absorption of serum protein in the kidney. Proteinuria is commonly seen in a variety of kidney diseases involvement, including lupus nephritis (LN), nephrotic syndromes, diabetic nephropathy, etc. In these cases, along with the endogenous serum protein, the therapeutic protein such as monoclonal antibody (mAb) is also detected in urine. Additional renal clearance of mAb may lead to a clinically important reduction in serum drug concentration.
Methods: We evaluated longitudinal pharmacokinetic and spot urine protein data for three monoclonal antibodies: mAb A in systemic lupus erythematosus (SLE), mAb B in ANCA-associated vasculitis (AAV), and mAb C in sickle cell disease (SCD). Two studies (in AAV and SCD) collected qualitative urine protein (i.e. dipstick) values, and two studies in SLE collected quantitative urine protein information (i.e., total Urine Protein to Creatinine Ratio, UPCR). The relationship between drug exposure and quantitative UPCR was assessed by fitting a linear model on log-transformed and normalized variables. The assumption of linearity was assessed using a loess-smoothed overlay.
Results: For the SLE studies (mAb A), the median baseline of UPCR (g/g) was 0.144 (IQR: 0.092 – 0.355), and 216 subjects had greater than 500 mg/g (n=1128). In the SCD study (mAb C), a majority of subjects (100/150) had negative protein dipstick readings at baseline, and 50/150 had detectable urine protein. In the AAV study (mAb B), 25/64 had negative protein dipstick readings, and 39/64 were positive for trace to 4+ protein dipstick values.
A statistically persuasive negative correlation between quantitative urine protein with total drug exposure was identified for mAb A in lupus patients (p < 0.00001).
The relationship between baseline urine UPCR and drug exposure was well-described by a linear model with logarithmic transformations. This model suggests, on average, a patient with 0.5 g/g UPCR would be expected to have 84% of the exposure (95% CI – 82-86%) of a subject with spot protein of 0.1 g/g or less. Likewise, a subject with 5 g/g would be expected to have 68% (95% CI: 64-73%) of the exposure of a subject with 0.1 g/g or less. Additionally, preliminary analyses suggest that the pre-dose trough concentration (a key covariate determining adequate dosing interval) may be more affected than overall exposure.
In contrast, no relationship was observed between qualitative urine protein and drug clearance for mAb B in AAV or mAb C in SCD. This may be due to the nature of the disease or the underlying pathophysiology of the kidney involvement, the degree of proteinuria, or other factors.
Conclusion: Overall, different types and severity of proteinuria may have different impact on the clearance of therapeutic proteins. The clinical impact of these observations remains to be determined. These findings may warrant the assessment, during drug development, of the impact of proteinuria on efficacy, safety, or the need for dosing adjustment for biological proteins.
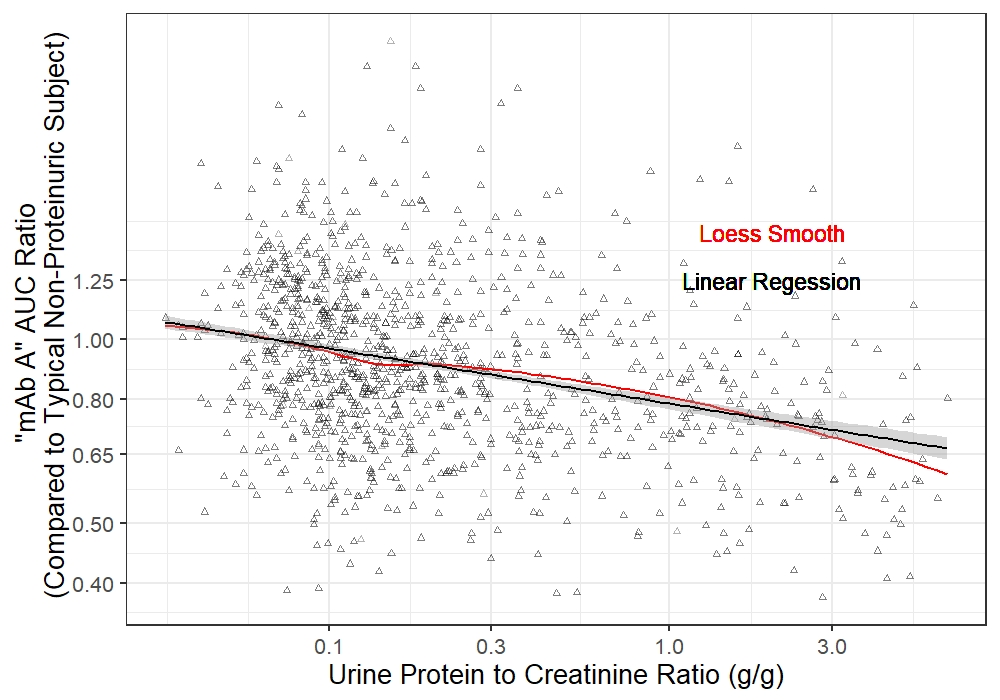 Figure 1. The relationship between baseline urine UPCR and mAb A exposure (AUC) in SLE patients. The black line represents linear regression, and the red line represents Loess smooth fitting.
Figure 1. The relationship between baseline urine UPCR and mAb A exposure (AUC) in SLE patients. The black line represents linear regression, and the red line represents Loess smooth fitting.
To cite this abstract in AMA style:
Penzenstadler J, Chen J, Park A, Neuner R, Thompson A, He L, Ji P, Nikolov N, Sahajwalla C. Impact of Proteinuria on the Clearance of Monoclonal Antibodies: Potential Clinical Implications [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/impact-of-proteinuria-on-the-clearance-of-monoclonal-antibodies-potential-clinical-implications/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-proteinuria-on-the-clearance-of-monoclonal-antibodies-potential-clinical-implications/
