Session Information
Date: Sunday, November 12, 2023
Title: (0460–0479) Reproductive Issues in Rheumatic Disorders Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: There are limited data on the impact of the postpartum period (PP) on the activity of inflammatory rheumatic disorders (IRD), and the data available on the impact of breastfeeding on the activity of IRD are contradictory. The objectives of this analysis were to describe disease activity and treatments received during the PP period and breastfeeding frequency and its possible impact on IRD activity.
Methods: A descriptive study from a French multicenter prospective observational cohort (GR2) studying pregnant women with IRD i.e. rheumatoid arthritis (RA) and spondylarthritis (SpA) confirmed by a rheumatologist was conducted (NCT 02450396). Data were collected during several regular pre-conception visits, visits during pregnancy and visits around 6- and 12-months PP, from October 2014 to October 2022. Disease activity was assessed with DAS28-CRP for RA and BASDAI and ASDAS for SpA. Flares were defined according to physician judgment. The change in disease activity was investigated by paired Student’s t-tests and Mac-Nemar Chi-2 tests for paired data, as appropriate. Subgroup bivariate comparisons by IRD pathology were performed according to patients’ breastfeeding status at 6-months PP by Kruskal-Wallis tests for continuous variables and Fisher exact tests for categorical variables. There was no imputation of missing data.
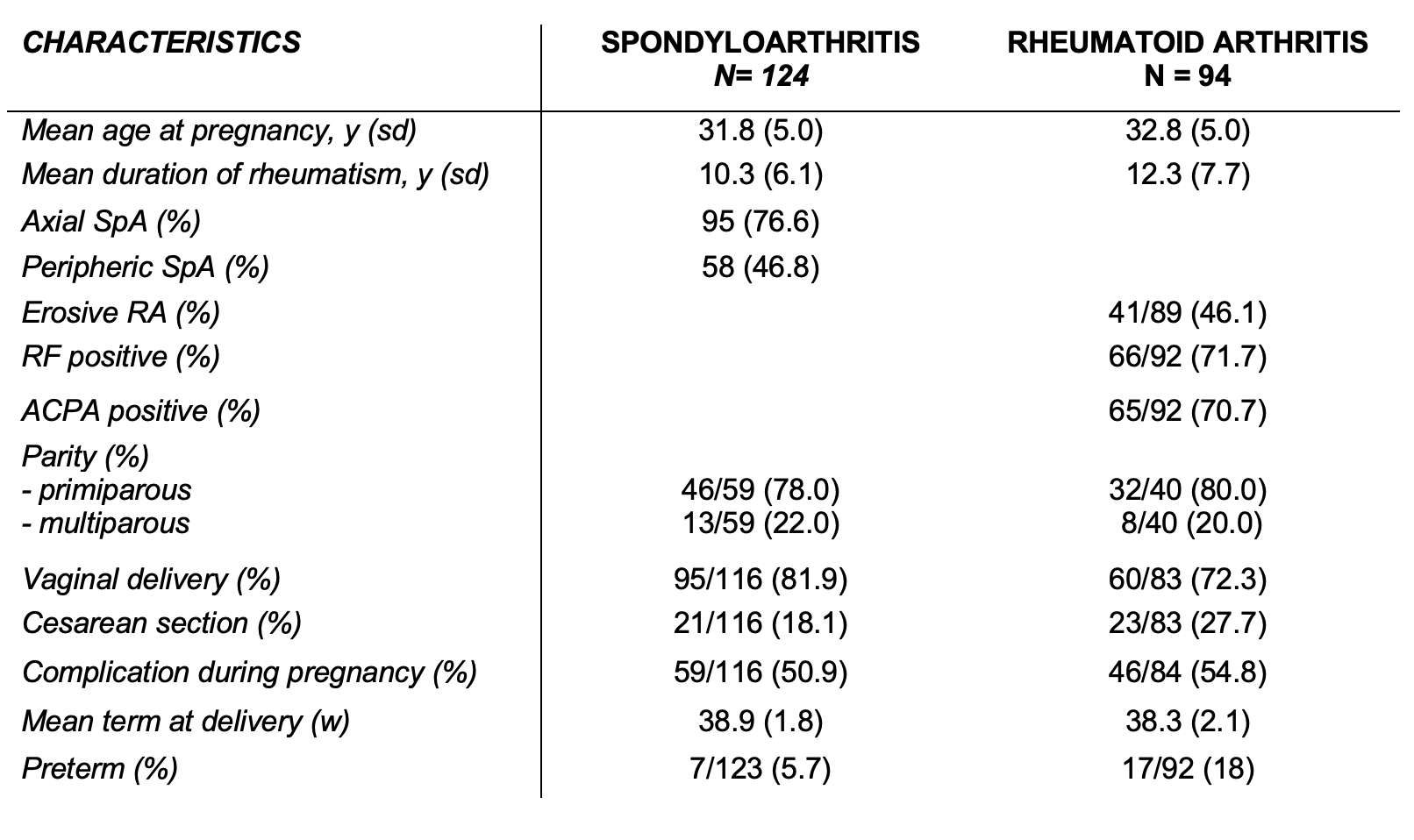
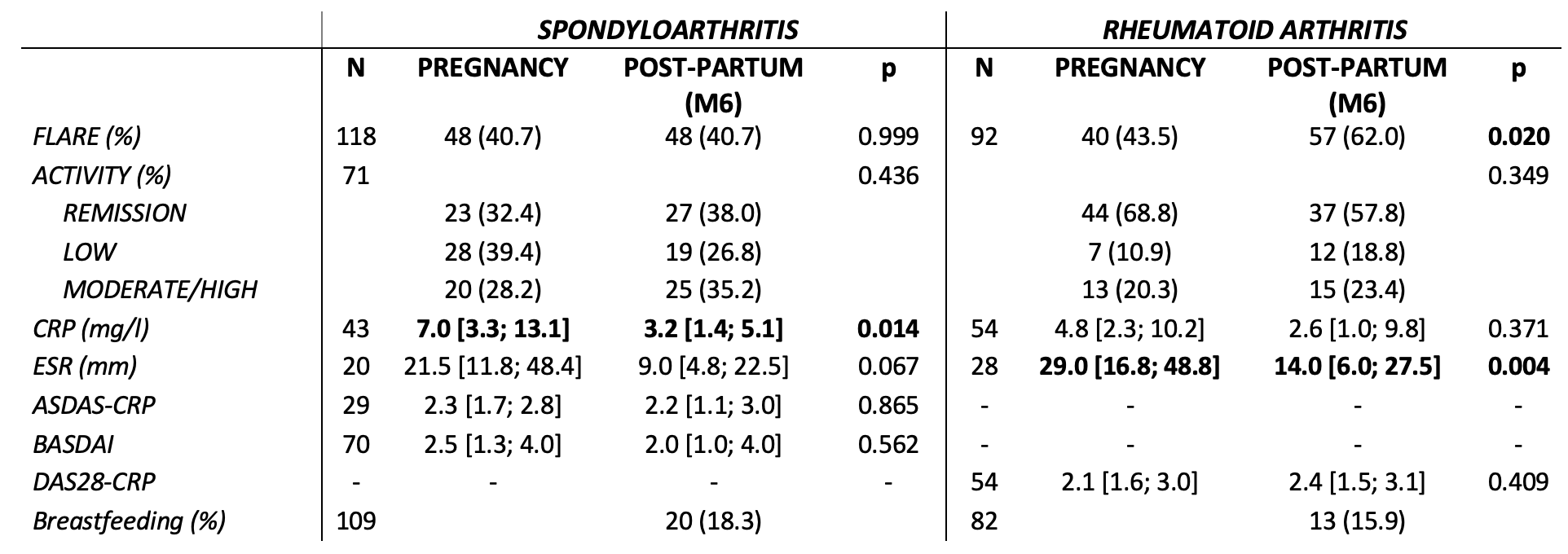
Results: Overall, 218 pregnant patients were analyzed: 124 with SpA and 94 with RA. The mean age was 31.8 ± 5.0 and 32.8 ± 5.0 years, respectively for SpA patients and RA patients (Table 1). In SpA patients, there were no significant differences in terms of activity (flares, BASDAI and ASDAS) at 6 months PP compared to during pregnancy. However, CRP level was significantly higher during pregnancy compared to 6 months PP (7 mg/L vs 3.2 mg/L; p = 0.014) (Table 2). RA patients presented significantly more flares in the first 6 months PP compared to pregnancy (62.0% vs 43.5% p = 0.02).Disease activity measures were similar between both SpA and RA patients who still breastfed at six months PP and patients who never breastfed or stopped before 6 months.
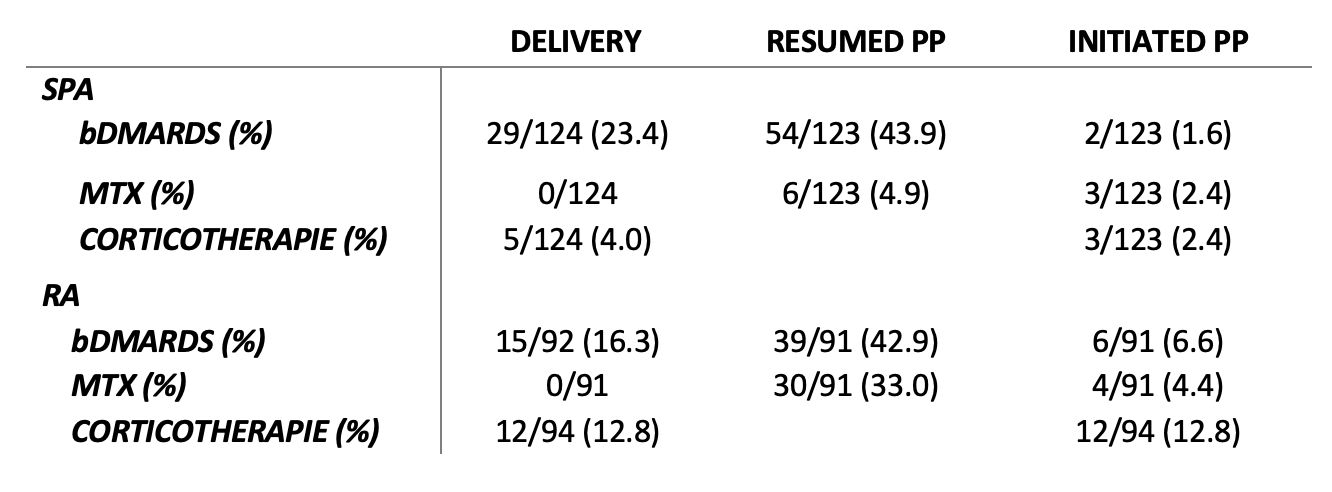
After one-year PP, among SpA patients, the number of flares were significantly higher than during pregnancy (40.3% vs 13.4%; p < 0.001). For RA patients, the mean activity and the number of flares were not different during pregnancy and at one year PP. Regarding treatment, about 40% of SpA and RA patients resumed bDMARD during PP, mainly in the first month PP (Table 3). For SpA, all bDMARDs were TNF inhibitors except for one treatment with belimumab (patient with SpA and systemic lupus erythematosus). For RA, among bDMARDs prescribed, 38/45 (84.4%) were TNF inhibitors (tocilizumab, abatacept and JAK inhibitors were the remaining).
Conclusion: RA patients presented significantly more flares in the first 6 months of postpartum compared to pregnancy while SpA patients remained stable. Yet, in both diseases, only about 40% of the patients resumed bDMARD during post-partum period. Breastfeeding was not associated with an increase or decrease in disease activity in SpA and RA patients.
To cite this abstract in AMA style:
Hornez M, Molto A, Leroy M, banneville b, Belkhir R, chauvet e, Couderc M, Dernis E, Desouches s, Devauchelle V, Frazier-Mironer A, Gossec L, Gervais e, Marotte H, Richez C, SELLAM J, Seror R, Le Guern V, Guettrot-Imbert G, Costedoat-Chalumeau N, Flipo R, Letarouilly J. Impact of Postpartum on Patients with Inflammatory Rheumatic Diseases: The P2Rheum Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/impact-of-postpartum-on-patients-with-inflammatory-rheumatic-diseases-the-p2rheum-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-postpartum-on-patients-with-inflammatory-rheumatic-diseases-the-p2rheum-study/