Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Evidence suggests that response to treatment in PsA may vary with patient (pt) characteristics, such as sex.1 This post hoc analysis of the Phase 3 SELECT-PsA 1 trial (NCT03104400) evaluated responses to the Janus kinase inhibitor upadacitinib (UPA) vs the TNF inhibitor adalimumab (ADA) at Weeks (Wks) 24 and 56 in selected pt subgroups.
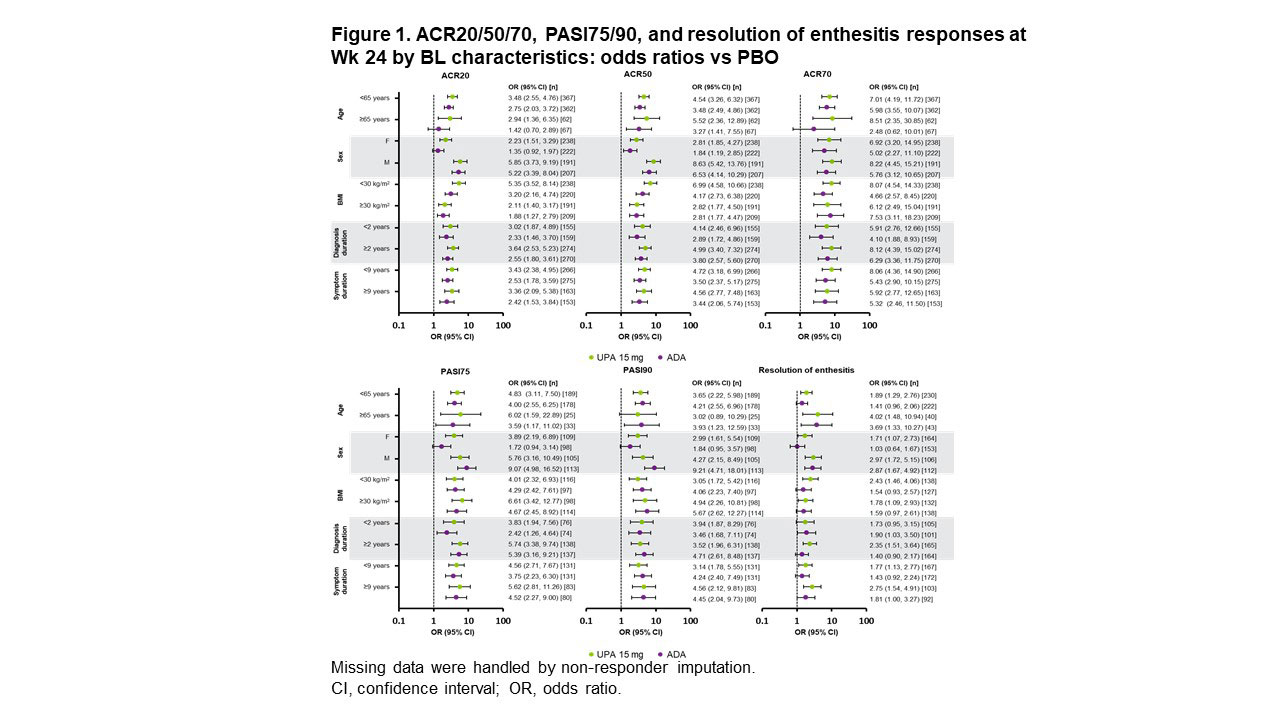
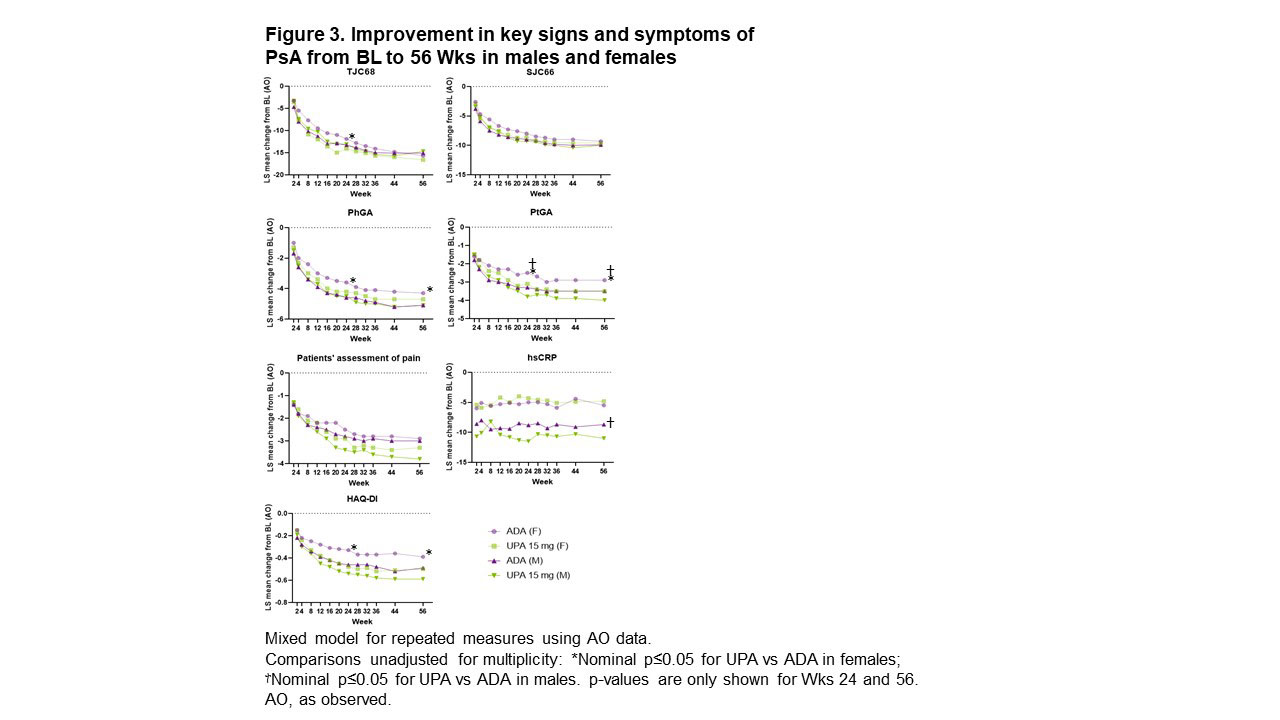
Methods: Pts in SELECT-PsA 1 were randomized 1:1:1:1 to UPA 15/30 mg once daily, ADA 40 mg every other wk, or placebo (PBO) followed by UPA 15/30 mg starting at Wk 24; treatment was double blinded until Wk 56.2 This subgroup analysis evaluated the efficacy of UPA 15 mg vs ADA based on pts’ ages (< 65 vs ≥65 years), sex (male [M] vs female [F]), BMI (< 30 vs ≥30 kg/m2), time since diagnosis (< 2 vs ≥2 years), and symptom duration (< 9 vs ≥9 years) at baseline (BL). Outcomes assessed included ≥20%/50%/70% improvement in ACR criteria (ACR20/50/70), ≥75%/90% improvement in Psoriasis Area and Severity Index (PASI75/90), and resolution of enthesitis, all at Wks 24 and 56; and change from BL in tender/swollen joint count in 68/66 joints (TJC68/SJC66), Physicians’ and Pts’ Global Assessment of disease activity (PhGA and PtGA), pts’ assessment of pain, high-sensitivity CRP (hsCRP), and Health Assessment Questionnaire Disability Index (HAQ-DI) through Wk 56.
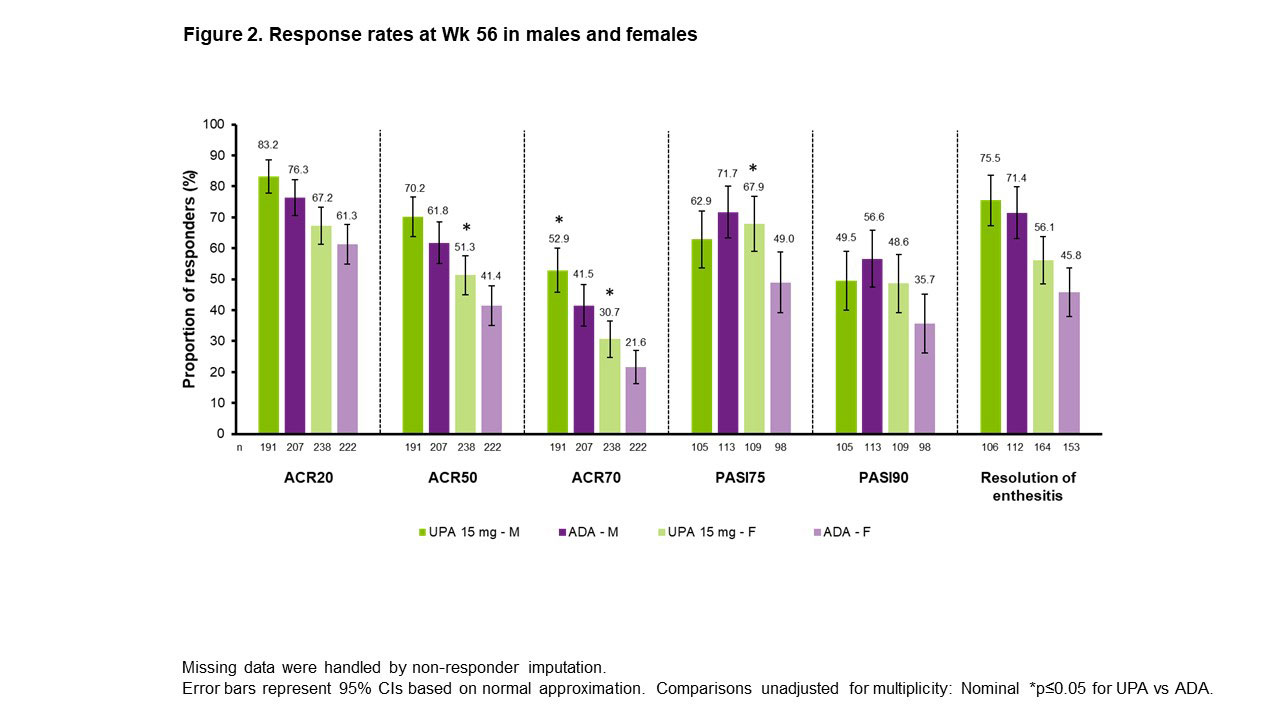
Results: ACR20/50/70 response rates at Wk 24 were significantly greater with UPA vs PBO across all subgroups evaluated, but not across all subgroups with ADA vs PBO (Figure 1). For ACR20/50, treatment effect sizes differed by sex and BMI, with greater differences seen in M vs F and in pts with lower (< 30 kg/m2) vs higher BMI. ACR20/50/70 response rates at Wk 56 were either comparable or higher with UPA vs ADA in both sexes (Figure 2). Rates of resolution of enthesitis at Wk 56 were comparable between UPA vs ADA in both sexes, but tended to be higher in M vs F. Between-sex differences in Wk 56 PASI75/90 response rates were minimal with UPA, but notably higher rates were seen in M vs F treated with ADA, with significantly higher PASI75 response rates in F with UPA vs ADA. Changes from baseline in TJC68/SJC66 were generally comparable between sexes in both treatment groups (Figure 3). Mean hsCRP levels at BL were higher in M (UPA, 13.5; ADA, 12.4) vs F (UPA, 9.0; ADA, 9.3), and generally greater mean changes in hsCRP from BL to Wk 56 were observed for M vs F within treatment groups. Generally greater mean changes were also observed in HAQ-DI from BL to Wk 56 for M vs F within treatment groups. The same was true of PhGA and PtGA, albeit to a lesser extent. Pts’ assessment of pain at BL was lower for M (UPA, 5.8; ADA, 5.4) vs F (UPA, 6.5; ADA, 6.5). Significantly greater improvements from BL were seen at Wk 56 with UPA vs ADA in PhGA, PtGA, and HAQ-DI in F, and in PtGA, pts’ assessment of pain, and hsCRP in M.
Conclusion: We observed sex-based differences in response to UPA vs ADA in pts with PsA. Significantly greater improvements in ACR70, PtGA, pts’ assessment of pain, and hsCRP in M, and in ACR50/70, PASI75, PhGA, PtGA, and HAQ-DI in F, were seen with UPA vs ADA at Wk 56.
References
1. Coates LC, et al. Lancet Rheumatol 2022;4:E262–73.
2. McInnes IB, et al. N Engl J Med 2021;384:1227–39.
To cite this abstract in AMA style:
Eder L, Coates L, Nash P, Kiltz U, Lubrano E, McDearmon-Blondell E, Gao T, Ogdie A. Impact of Patient Characteristics, Including Sex, on the Efficacy of Upadacitinib Compared with Adalimumab in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/impact-of-patient-characteristics-including-sex-on-the-efficacy-of-upadacitinib-compared-with-adalimumab-in-patients-with-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-patient-characteristics-including-sex-on-the-efficacy-of-upadacitinib-compared-with-adalimumab-in-patients-with-psoriatic-arthritis/