Session Information
Date: Sunday, October 26, 2025
Title: (0233–0279) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Idiopathic hypereosinophilic syndromes (iHES) are a group of disorders characterized by persistent eosinophilia and eosinophil-mediated organ damage. Mepolizumab, an anti-interleukin-5 monoclonal antibody, has proven effective in reducing eosinophilic burden, controlling clinical manifestations in various forms of iHES, and decreasing the reliance on glucocorticoids (GCs). This study aims to evaluate the clinical and steroid-sparing efficacy of mepolizumab in a monocentric retrospective cohort of patients with iHES.
Methods: We analyzed a cohort of patients diagnosed at our Center according to the 2023 refined diagnostic criteria and classification for HES (1). Only patients diagnosed with iHES and treated with mepolizumab were included. Descriptive statistics included medians (1st-3rd IQR). Differences were assessed using the Wilcoxon test (significance: p ≤ 0.05). Variables were analyzed immediately prior to mepolizumab initiation (t0) and at 1 (t1), 3 (t3), 6 (t6), 12 (t12), 18 (t18), and 24 (t24) months post-treatment.
Results: Seven patients were enrolled [3 males, 4 females; median age at HES onset: 49 (35-59) years; median age at diagnosis: 49 (37-59) years; median diagnostic delay: 4 (1-26) months]. Clinical features at onset are reported in Table 1. At HES onset, median eosinophil count (EC) was 6910 (5685-16525) cells/μl and all patients showed hypereosinophilia. The median time from diagnosis to mepolizumab initiation was 5 (1-18) months, with a median follow-up of 24 (12-24) months. Six patients had received GCs prior to mepolizumab and were still on steroid therapy at the time of initiation. Furthermore, patient 2 had been treated with cyclosporine A for lichenified trunk eczema. At t0, 1 patient showed eosinophilia and 3 hypereosinophilia. A marked decrease in EC was observed as early as t1 [median t0: 810 (438-1250) cells/μl; median t1: 120 (106-125) cells/μl; p: 0.0156], which was maintained at the last available follow-up [median at last follow-up: 70 (30-625) cells/μl; p: 0.0469]. At t6, 2 patients showed a significant rebound in ECs. In patient 1, hypereosinophilia persisted from t6 onward, and both GCs and dupilumab were introduced at t18. Patient 6 was hospitalized at t6 and later died from septic shock (notably, with a history of substance abuse) (Table 2; Graphic 1). Overall, the median percentage reduction in EC was 89 (78-92) % (p: 0.0469). During follow-up, GCs were discontinued in 5 patients and later reintroduced in 3 cases due to HES relapses. The comparison of GC daily dose at t0 and at the last available follow-up showed a significant reduction [median at t0: 27 (2-51) mg/day; median at last follow-up: 0 (0-4) mg/day; p: 0.0469] (Table 2). The number of HES relapses before (n: 9) and after (n: 4) initiation of mepolizumab showed a trend toward statistical significance, although not reaching it (p: 0.0780).
Conclusion: Preliminary findings from our cohort suggest that mepolizumab may be effective in achieving clinical control and reducing GC dependence in patients with iHES.References:(1) Valent P, et al. Proposed refined diagnostic criteria and classification of eosinophil disorders and related syndromes. Allergy. 2023 Jan;78(1):47-59
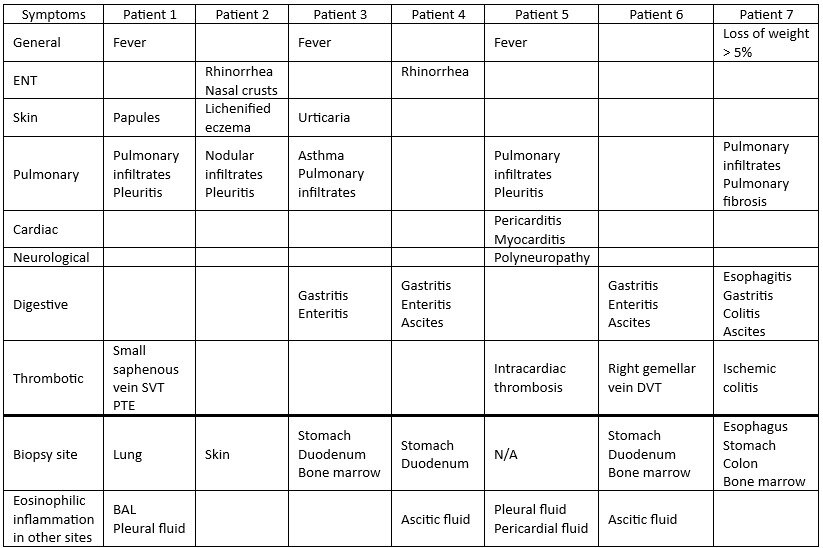 Table 1. Clinical manifestations from iHES onset to diagnosis. Abbreviations: ENT = Ear, Nose, and Throat; SVT = superficial venous thrombosis; PTE = pulmonary thromboembolism; BAL = bronchoalveolar lavage; DVT = deep venous thrombosis.
Table 1. Clinical manifestations from iHES onset to diagnosis. Abbreviations: ENT = Ear, Nose, and Throat; SVT = superficial venous thrombosis; PTE = pulmonary thromboembolism; BAL = bronchoalveolar lavage; DVT = deep venous thrombosis.
.jpg) Table 2. Eosinophil count and glucocorticoid daily dosage at t0 (immediately prior to mepolizumab initiation) and at different time points (each representing the number of months) after mepolizumab initiation.
Table 2. Eosinophil count and glucocorticoid daily dosage at t0 (immediately prior to mepolizumab initiation) and at different time points (each representing the number of months) after mepolizumab initiation.
.jpg) Graphic 1. Eosinophil count trend over time, from t0 (immediately prior to mepolizumab initiation) to t24. For Patient 5, an outlier value of 25010 cells/μl was excluded to improve graphical readability; however, the overall trend line was maintained to reflect the general trajectory.
Graphic 1. Eosinophil count trend over time, from t0 (immediately prior to mepolizumab initiation) to t24. For Patient 5, an outlier value of 25010 cells/μl was excluded to improve graphical readability; however, the overall trend line was maintained to reflect the general trajectory.
To cite this abstract in AMA style:
Mora J, Regola F, Toniati P, Fontana G, Gatti A, Cavazzana I, Franceschini F. Impact of Mepolizumab on Idiopathic Hypereosinophilic Syndromes: Preliminary Analysis of a Monocentric Retrospective Cohort [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-of-mepolizumab-on-idiopathic-hypereosinophilic-syndromes-preliminary-analysis-of-a-monocentric-retrospective-cohort/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-mepolizumab-on-idiopathic-hypereosinophilic-syndromes-preliminary-analysis-of-a-monocentric-retrospective-cohort/
