Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: International guidelines recommend that in psoriatic arthritis infliximab should be dosed with 5 mg/kg bodyweight every 8th week. Data on the use of lower doses is however scarce. We aimed to describe dose regimens, the frequency of dose escalation and outcomes in patients with psoriatic arthritis treated with infliximab in routine care.
Methods: Observational cohort study based on the nationwide Danish DANBIO and Icelandic ICEBIO registries. Demographics and baseline characteristics among tumor-necrosis-factor-α inhibitor (TNFi) naïve patients treated with ≤3 mg infliximab/kg body weight, 3-5 mg/kg or ≥5 mg/kg with treatment scheduled at 0, 2, 6 and then every 8th week were described. Dose escalation was defined as an increase in either the dose and/or frequency. Treatment responses were evaluated by ACR20/50/70 and EULAR-good-response after 6 months’ treatment and disease activity after one year’s treatment. Kaplan-Meier plots and regression analyses were performed for drug survival analyses and to identify predictors of treatment response and drug survival.
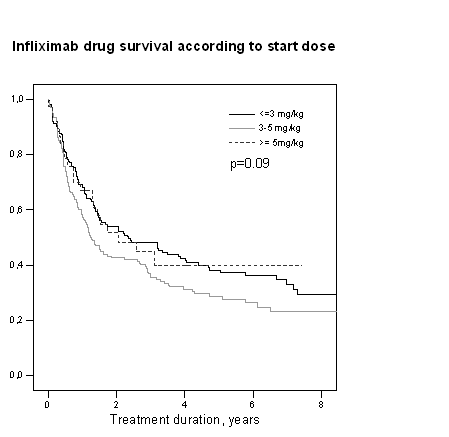
Results: Among the 1589 psoriatic arthritis patients identified in the registries, 462 patients (29%, 376 Danish, 86 Icelandic) received treatment with infliximab. Start infliximab dose was ≤3 mg/kg in 174 patients (38%), 3-5 mg/kg in 174(38%), ≥5mg/kg in 38(8%) and unregistered in 76 patients (16%). After one years’ treatment, corresponding percentages were 35, 53, 12 and 22%, respectively. Patients with higher body weight received lower infliximab doses per kg. The median time until first dose escalation (273 days (interquartile range, IQR 153-553) was independent of start dose (log rank 0.1, p=0.9). The ACR20/50/70 or EULAR-response rates, drug survival (Figure) and one-year disease activity were also independent of baseline dose. Icelandic patients received lower infliximab doses than Danish (2.3mg/kg (2.1-2.9) vs. 3.1mg/kg (3.0-3.8)) (median (IQR), p<0.05), they had also longer drug survival, but similar one-year response rates.
Conclusion : In clinical practice, >80% of infliximab treated patients with psoriatic arthritis received sustained treatment with doses less than the 5 mg/kg/8weeks recommended in international guidelines. Low infliximab start dose did not affect drug survival or treatment response. Low start dose with gradual dose escalation was a preferred and effective strategy.
Figure
|
|
Disclosure:
B. Glintborg,
None;
B. Gudbjornsson,
None;
N. S. Krogh,
None;
E. Omerovic,
None;
N. Manilo,
None;
M. Holland-Fischer,
UCB, MSD, Roche,
8,
UCB, MSD, Roche,
5;
H. M. Lindegaard,
Lilly, MSD, Nordpharma, Roche Pharmaceuticals,
2,
Roche Pharmaceuticals, MSD,
5;
A. G. Loft,
MSD,
6,
MSD,
8;
H. Nordin,
None;
L. Johnsen,
None;
S. Flejsborg Oeftiger,
None;
A. Hansen,
UCB, ABBVIE, MSD ,
8;
C. Rasmussen,
Pfizer, Abbvie og Vertex,
2,
Abbvie og Pfizer.,
8;
G. Grondal,
None;
J. Geirsson,
None;
M. L. Hetland,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-low-infliximab-dose-regimen-on-treatment-response-and-drug-survival-in-462-patients-with-psoriatic-arthritis-results-from-the-nationwide-registries-danbio-and-icebio/