Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Chronic pain associated with rheumatoid arthritis (RA) leads to impaired patient function and quality of life. Prior studies suggest initiation of disease-modifying antirheumatic drugs (DMARDs) is associated with RA pain relief. However, real-world evidence on the impact of biologic and targeted synthetic DMARDs (bDMARDs and tsDMARDs) on pain treatments is limited. This study aims to assess pain medication use before vs. after bDMARD or tsDMARD initiation in RA patients in a real-world setting.
Methods: Using Optum Clinformatics data (2009-20), we conducted a cohort study with 4 groups of adult RA patients who were first- or second-line initiators of bDMARDs or tsDMARDs. Patients were required to have ≥one-year continuous insurance enrollment before and after cohort entry (i.e., bDMARD or tsDMARD initiation date), without pregnancy, cancer, kidney transplant, or dialysis at baseline (i.e., one year before cohort entry), and without initiating >1 bDMARD or tsDMARD at cohort entry. Our study outcomes include use of 5 medication classes for pain management (NSAIDs/COX-2 inhibitors, opioids, oral corticosteroids, gabapentinoids, and muscle relaxants), use of 6 subclasses of opioids, and daily prednisone and morphine equivalent dose among oral corticosteroid and opioid users. Using intention-to-treat analysis, study participants were followed one year from one day after cohort entry.
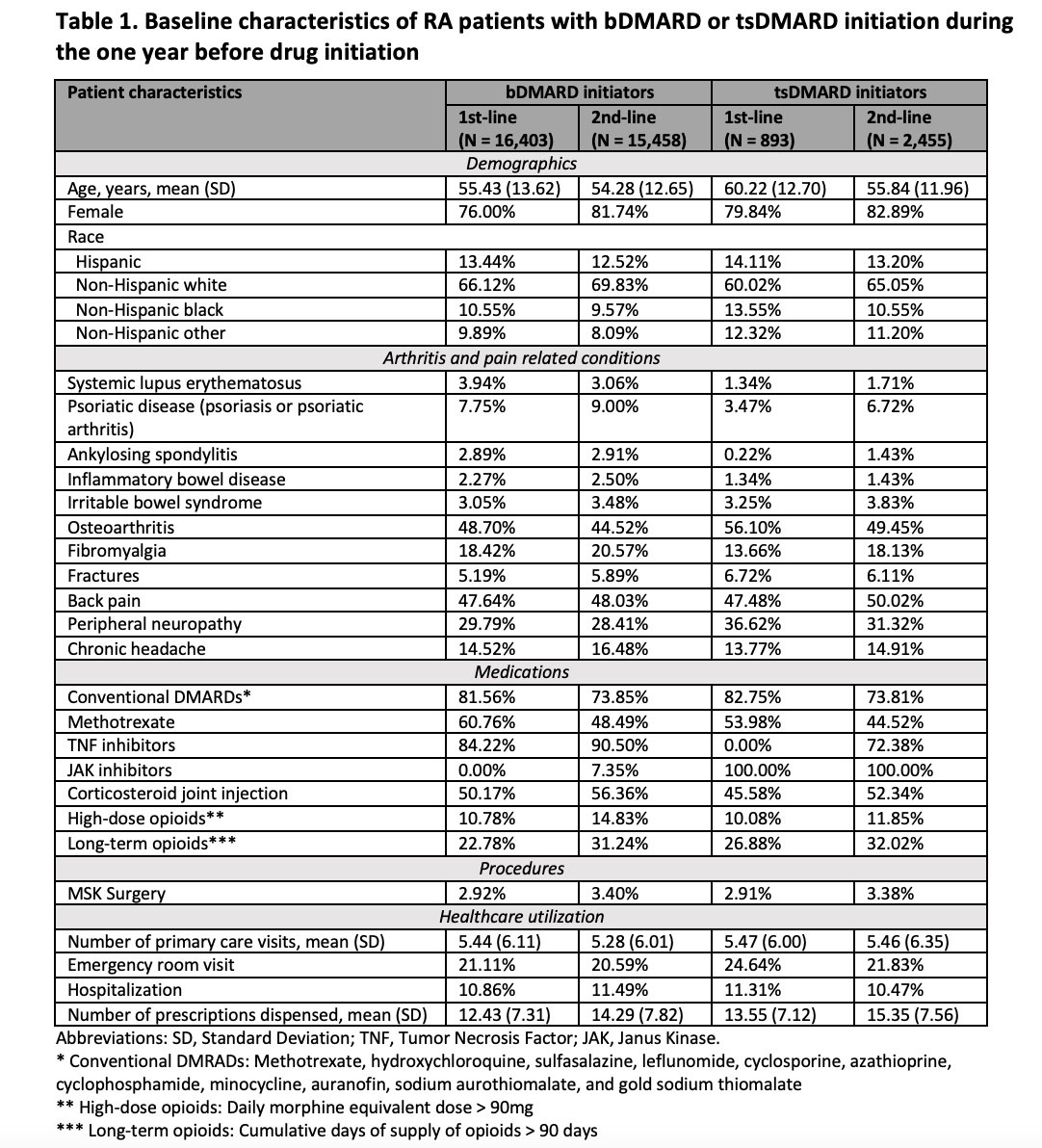
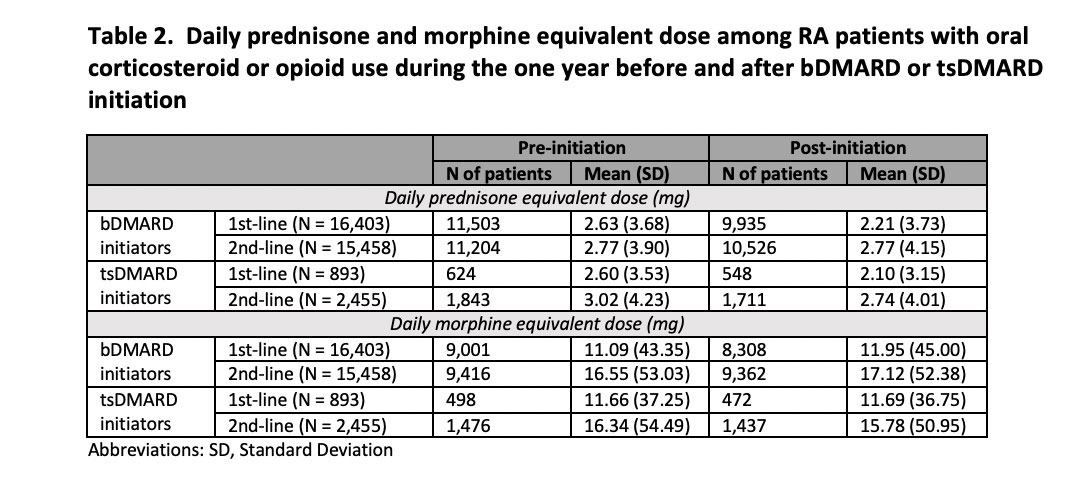
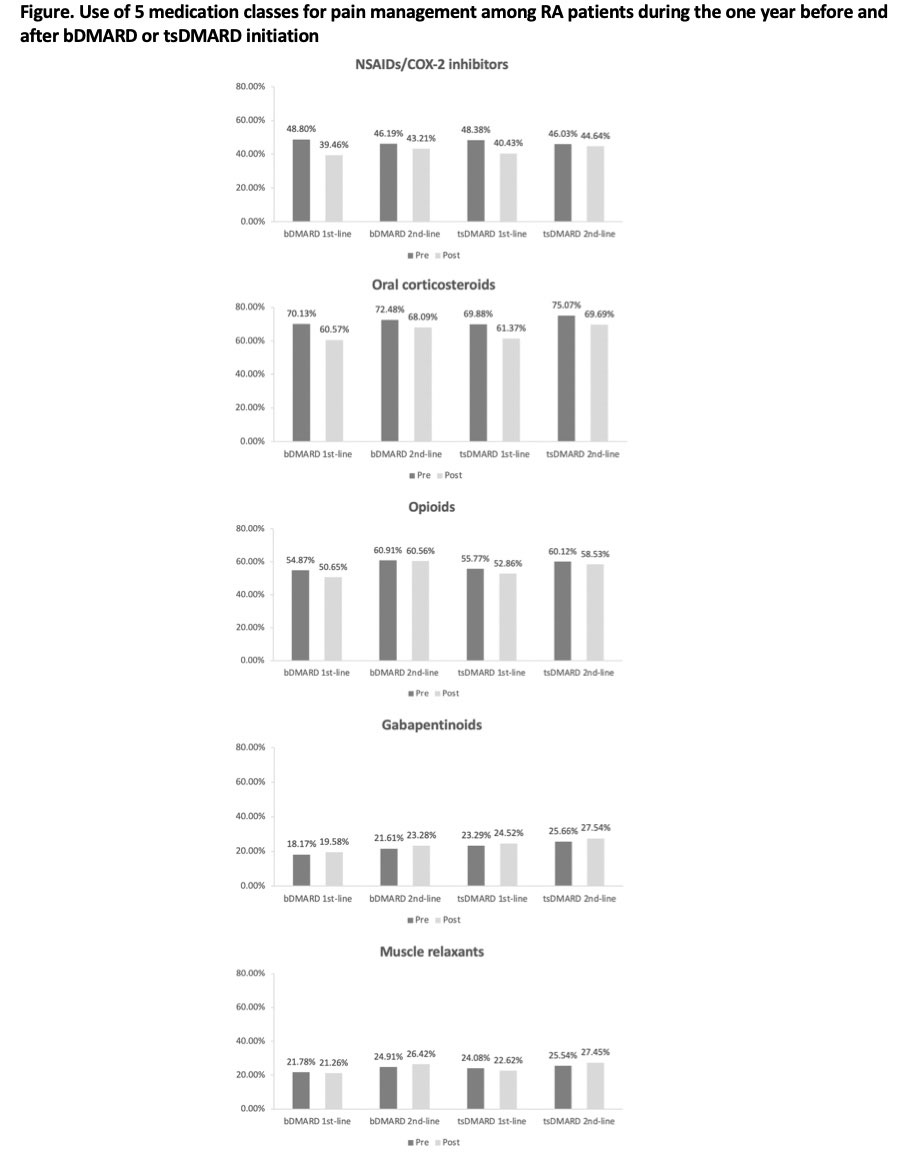
Results: The study cohort included 16,403 bDMARD first-line initiators, 15,458 bDMARD second-line initiators, 893 tsDMARD first-line initiators, and 2,455 tsDMARD second-line initiators. Their mean (standard deviation) age ranged between 54.28 (12.65) and 60.22 (12.70) years. 76.00-82.89% were female. Patients in all 4 groups had high prevalence of osteoarthritis, corticosteroid joint injections, and high-dose and long-term opioid use at baseline (Table 1). Before initiating bDMARDs or tsDMARDs, the prevalence of pain medication use in first-line initiators was 46.19-48.80% for NSAIDs/COX-2 inhibitors, 70.13-72.48% for oral corticosteroids, 54.87-60.91% for opioids, 18.17-21.61% for gabapentinoids, and 21.78-24.91% for muscle relaxants. Within one year after initiation, the prevalence decreased by 7.95-9.35% for NSAIDs/COX-2 inhibitors, 8.51-9.56% for oral corticosteroids, 2.91-4.22% for opioids, and 0.52-1.46% for muscle relaxants, but increased by 1.23-1.41% for gabapentinoids (Figure). Reduced opioid use was mainly driven by the decline in hydrocodone and tramadol use. Mean daily prednisone equivalent dose among oral corticosteroid users decreased from 2.60-2.63mg to 2.10-2.21mg while mean daily morphine equivalent dose among opioid users increased from 11.09-11.66mg to 11.69-11.95mg (Table 2). Changes were smaller though similar in second-line users.
Conclusion: Initiation of bDMARDs or tsDMARDs in RA patients is associated with decreased use of NSAIDs/COX-2 inhibitors, oral corticosteroids, opioids, and muscle relaxants, but increased use of gabapentinoids within one year post the drug initiation. Among oral corticosteroid and opioid users, bDMARD or tsDMARD initiation had very small but opposite impact on dose changes. The observed utilization patterns warrant further studies.
To cite this abstract in AMA style:
He M, Lee Y, Jin Y, Desai R, Vine S, Kim S. Impact of Initiating Biologic and Targeted Synthetic Disease-Modifying Antirheumatic Drugs on Pain Medication Use in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/impact-of-initiating-biologic-and-targeted-synthetic-disease-modifying-antirheumatic-drugs-on-pain-medication-use-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-initiating-biologic-and-targeted-synthetic-disease-modifying-antirheumatic-drugs-on-pain-medication-use-in-patients-with-rheumatoid-arthritis/