Session Information
Date: Monday, October 27, 2025
Title: Abstracts: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: Therapies (0873–0878)
Session Type: Abstract Session
Session Time: 10:15AM-10:30AM
Background/Purpose: The first-in-class, oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor deucravacitinib has an established clinical profile in moderate to severe plaque psoriasis with over 5 years of long-term data1 and is approved in multiple countries for this indication. Deucravacitinib showed superior efficacy vs placebo (PBO) at W16 across multiple endpoints in patients (pts) with active PsA in the global, randomized, double-blind, PBO-controlled phase 3 POETYK PsA-1 (NCT04908202) and PsA-2 (NCT04908189) studies, with no new safety signals.2,3 Composite efficacy measures provide a comprehensive assessment of disease activity and response to treatment across several disease domains. We report deucravacitinib efficacy based on composite measures of disease activity in pts from the POETYK PsA studies.
Methods: Pts in the POETYK PsA-1 and PsA-2 studies were randomized to deucravacitinib 6 mg once daily or PBO for 16 weeks (in PsA-2, a group received apremilast as a safety reference arm). This analysis included pts treated with deucravacitinib or PBO to W16. Composite measures of disease activity, including minimal disease activity (MDA), Disease Activity in Psoriatic Arthritis (DAPSA), Psoriatic Arthritis Disease Activity Score (PASDAS), modified Composite Psoriatic Disease Activity Index (mCPDAI), and Psoriatic Arthritis Response Criteria (PsARC), were analyzed using nonresponder imputation for missing binary data and control-based pattern imputation for missing continuous data.
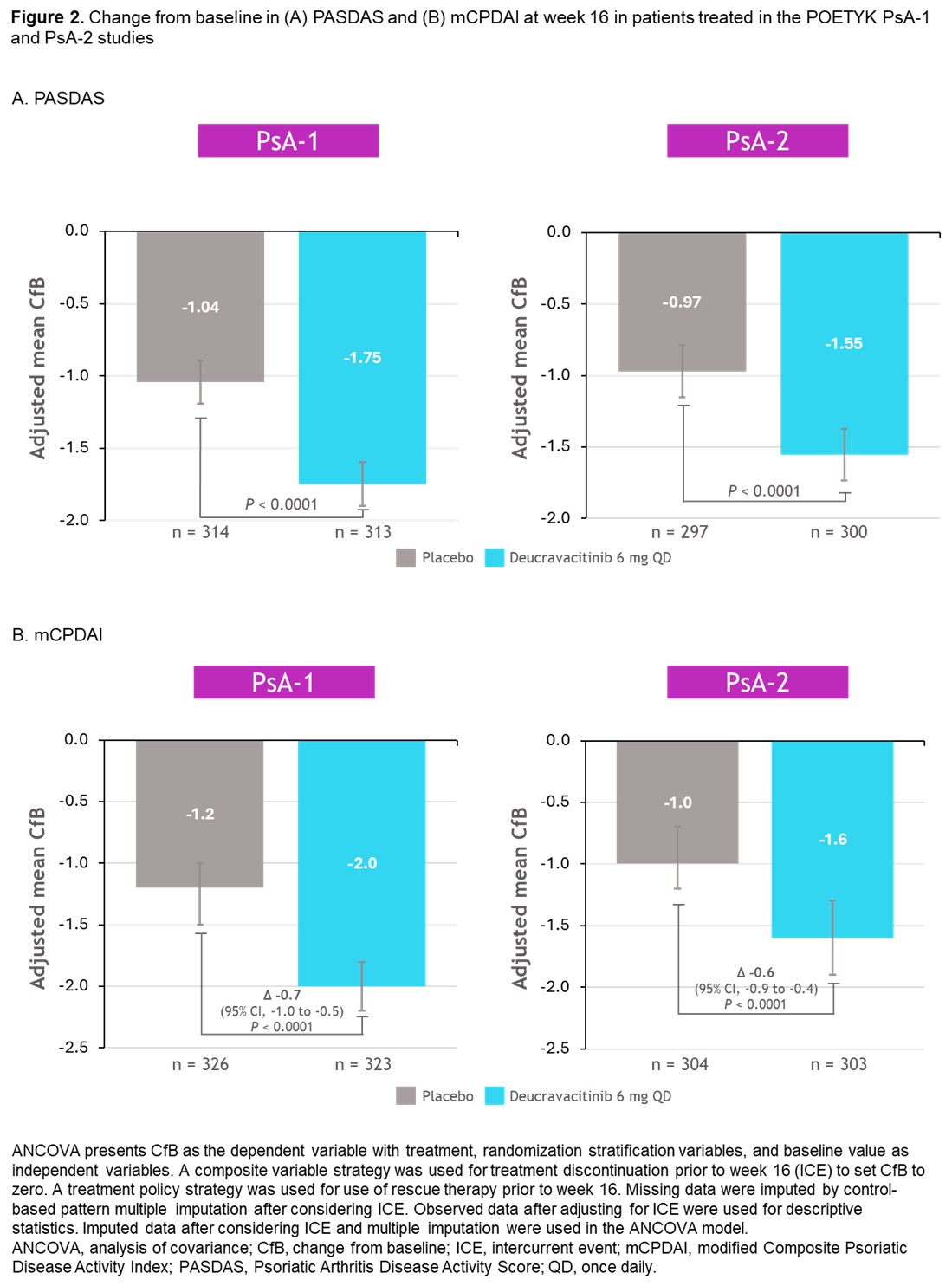
Results: At W16, a significantly higher proportion of pts treated with deucravacitinib vs PBO achieved MDA response (PsA-1, P = 0.0012; PsA-2, P = 0.0007), DAPSA low disease activity or remission (PsA-1, P = 0.0001; PsA-2, P < 0.0001), DAPSA disease remission (PsA-1, P = 0.0009; PsA-2, P = 0.0005), and PsARC response (PsA-1, P < 0.0001; PsA-2, P < 0.0001; Figure 1). Decrease from baseline in PASDAS and mCPDAI at W16 was significantly greater in pts treated with deucravacitinib vs PBO (PASDAS: PsA-1, P < 0.0001; PsA-2, P < 0.0001; mCPDAI: PsA-1, P < 0.0001; PsA-2, P < 0.0001; Figure 2). Efficacy was consistently higher with deucravacitinib vs PBO in male and female pts.
Conclusion:
Conclusion: In these analyses of the pivotal POETYK PsA-1 and PsA-2 phase 3 studies, deucravacitinib substantially reduced disease activity vs PBO at W16 in several composite measures of disease activity, and a significantly higher proportion of pts reached optimal control of disease activity, which aligns with the results from the primary analyses.2,3 Given that remission and low disease activity are primary goals of PsA treatment, deucravacitinib may be an efficacious treatment choice for pts with active PsA.ReferencesArmstrong AW, et al. J of Skin. 2025;9:s532.Mease PJ, et al. Presented at the 2025 American Academy of Dermatology Annual Meeting; March 7-11, 2025; Orlando, FL. Abstract 66894.Bristol Myers Squibb. News Release. Accessed April 21, 2025. https://news.bms.com/news/details/2024/Bristol-Myers-Squibb-Announces-Positive-Topline-Results-from-Two-Pivotal-Phase-3-Trials-Evaluating-Sotyktu-deucravacitinib-in-Adults-with-Psoriatic-Arthritis/default.aspx.
To cite this abstract in AMA style:
Deodhar A, Blanco R, Kavanaugh A, Gottlieb A, Coates L, Ritchlin C, Kivitz A, Zeng X, Morita A, Thaçi D, Varga S, Li K, Jou Y, Vritzali E, Mease P. Impact of Deucravacitinib on Disease Activity in Patients With Psoriatic Arthritis (PsA): Results From the Pivotal Phase 3 PsA Studies [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/impact-of-deucravacitinib-on-disease-activity-in-patients-with-psoriatic-arthritis-psa-results-from-the-pivotal-phase-3-psa-studies/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-deucravacitinib-on-disease-activity-in-patients-with-psoriatic-arthritis-psa-results-from-the-pivotal-phase-3-psa-studies/

.jpg)