Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: RA – Treatments III: Predictors of Response & Tapering
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Recent randomized-controlled studies have suggested that tapering bDMARDs and/or csDMARDs after achieving sustained remission in patients with rheumatoid arthritis (RA) can be considered. However, the optimal tapering strategy is yet to be established, and it may differ between various circumstances. We investigated whether concomitant treatment of methotrexate (MTX) may influence the disease activity after tapering abatacept in patients with RA.
Methods: We utilized the data from the Korean College of Rheumatology Biologics and Targeted therapy Registry (KOBIO-RA) (NCT01965132). Patients were enrolled when initiating a bDMARD (defined as baseline) and were followed up annually thereafter. Follow-up data on disease activity, concomitant medications, and dose of bDMARDs were collected. We included patients who 1) were treated with abatacept at baseline and 2) had no missing data on the dose of abatacept and MTX. The observational unit in this study was a 1-year interval. Patients were divided into two groups according to whether the dose of abatacept was de-escalated or not compared with the preceding interval (tapering group vs. control group). The primary outcome of the study was DAS28-ESR-based remission in the 1-year interval. To minimize confounding by indication between the two groups, we used the marginal structural model in which inverse probability of treatment weights (IPTWs) for each interval was applied to control all baseline and time-varying confounders.
Results: A total of 505 intervals from 179 patients were analyzed. The mean (SD) age of the patients was 59.3 (12.9) years and 150 (83.8%) were females. Baseline DAS28-ESR was 5.7 (1.0) and the mean (SD) weekly dose of MTX was 12.5 (3.3) mg. Tapering abatacept was undergone in 146 (28.9%) intervals, with a mean dose quotient of 0.68. The DAS28-ESR measured in the interval before tapering abatacept was 3.4 (1.3) and DAS28 remission was achieved in only 51 (34.9%) intervals before tapering abatacept.
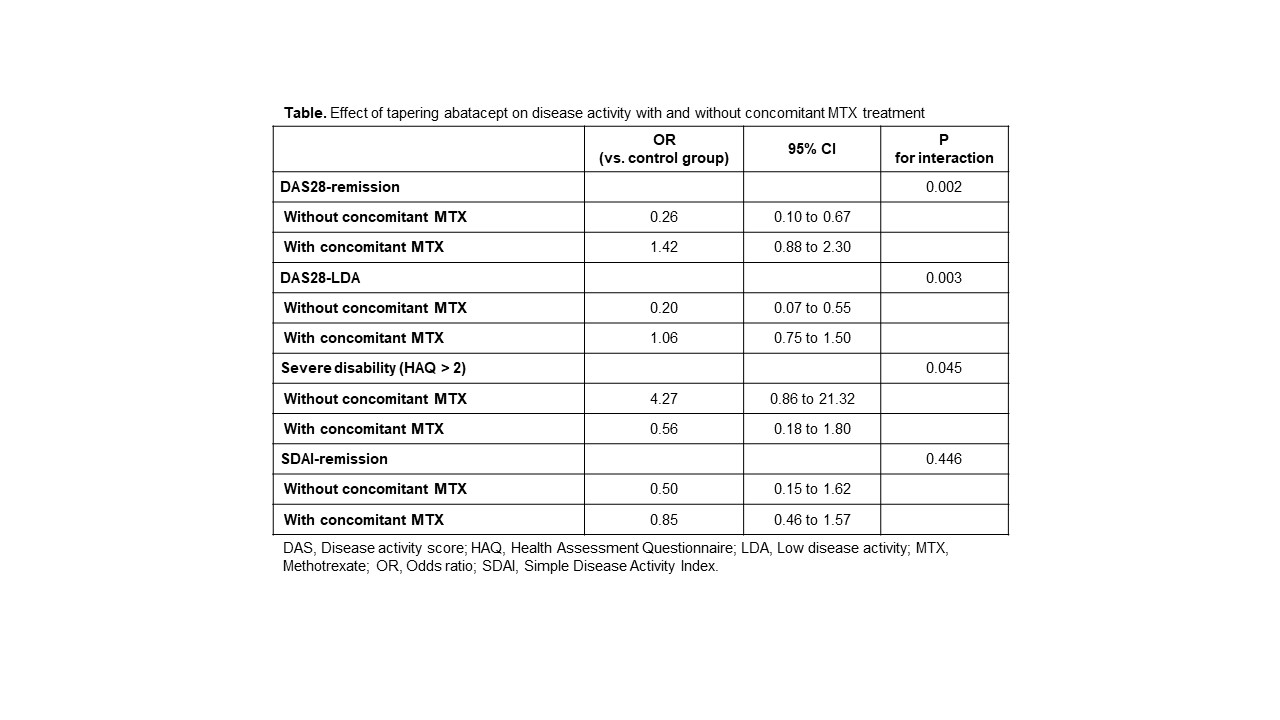
In the IPTW-applied pseudopopulation, the effect of tapering abatacept was significantly different based on concomitant use of MTX (P for interaction 0.002). In the subgroup of 1-year intervals without concomitant MTX, tapering abatacept significantly lower the odds for achieving DAS28-based remission, with an OR of 0.26 (95% CI 0.10 to 0.67). In contrast, with concomitant MTX, tapering abatacept did not have any significant effect (OR 1.42 [0.88 to 2.30]). This result was consistent when the effect of tapering abatacept was estimated using the outcome variable of achieving DAS28-low disease activity, or severe functional impairment (defined as HAQ >2). However, tapering abatacept with/without MTX did not significantly affect the odds for achieving SDAI-based remission (Table).
Conclusion: Tapering abatacept in the absence of concomitant MTX treatment is associated with a worse outcome in patients with RA.
To cite this abstract in AMA style:
Park J, Kim M, Kim H, Kim J, Shin K. Impact of Concomitant Methotrexate on Disease Activity After Tapering Abatacept in Patients with Rheumatoid Arthritis: A Nationwide Cohort Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/impact-of-concomitant-methotrexate-on-disease-activity-after-tapering-abatacept-in-patients-with-rheumatoid-arthritis-a-nationwide-cohort-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-concomitant-methotrexate-on-disease-activity-after-tapering-abatacept-in-patients-with-rheumatoid-arthritis-a-nationwide-cohort-study/