Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Glucocorticoid (GC) therapy has strong anti-inflammatory effects and helps slow radiographic progression in RA1; however, GCs can be associated with adverse events (AEs) such as infection, especially with long-term use and higher doses. This ad hoc analysis of three Phase 3 studies of upadacitinib (UPA) in RA (SELECT-NEXT, SELECT-MONOTHERAPY, and SELECT-EARLY) aimed to evaluate the impact of baseline GCs on the efficacy and safety of UPA with or without concomitant conventional synthetic DMARDs (csDMARDs).
Methods: Patients with inadequate response to MTX (MTX-IR) receiving UPA 15 mg once daily (QD) or placebo (PBO) + csDMARDs in SELECT-NEXT, and MTX-IR/MTX-naïve patients receiving UPA 15 mg QD monotherapy or MTX monotherapy in SELECT-MONOTHERAPY/SELECT-EARLY, respectively, were included in the analysis. Efficacy outcomes, including measures of remission and low disease activity (LDA) determined by DAS in 28 joints using CRP (DAS28[CRP]; < 2.6/≤3.2) and Clinical Disease Activity Index (CDAI; ≤2.8/≤10), were assessed and stratified by baseline GC use. Patients were permitted to receive oral GCs ≤10 mg/day (prednisone equivalent) at baseline with no adjustment permitted until Week 24/26/48. Safety was reported as number and proportion of patients with AEs. Data were analyzed descriptively with no statistical comparisons between groups or doses.
Results: Of 1,506 patients included in the analysis, 737 (48.9%) were receiving baseline GCs (mean dose 6.2 mg/day). Baseline characteristics were broadly similar across treatment groups; SELECT-EARLY, which enrolled MTX-naïve patients, generally had the shortest duration of RA and higher CRP levels. Across UPA treatment groups, concomitant GCs generally did not influence the proportions of patients achieving remission (Figure 1). In SELECT-NEXT, clinical responses with UPA 15 mg in combination with csDMARDs were similar irrespective of concomitant GC use (Figure 1). Within SELECT-MONOTHERAPY, responses in patients receiving UPA 15 mg without concomitant csDMARDs or GCs were higher than those in patients receiving MTX alone, but were numerically lower than in those receiving UPA 15 mg with GCs (Figure 1). However, this was not observed within SELECT-EARLY, where clinical responses in patients receiving UPA 15 mg monotherapy without GCs were higher than in those patients receiving UPA 15 mg with GCs for both DAS28(CRP) < 2.6 (40.6% vs 29.9%, respectively) and CDAI ≤2.8 (20.0% vs 11.6%, respectively) (Figure 1). A similar trend was observed for LDA. Serious AEs, AEs leading to discontinuation, and AEs of special interest, including infections (such as herpes zoster), were broadly similar in the UPA groups irrespective of concomitant GC use (Table 1).
Conclusion: UPA 15 mg in combination with csDMARDs or as monotherapy was effective in achieving remission and LDA, irrespective of concomitant GC use. Safety of UPA, including incidence of infection, appeared largely unaffected by concomitant GC use.
- Kirwan JR, et al. Cochrane Database Syst Rev 2007;1:CD006356.
 ᵃIncludes non-treatment-emergent deaths. ᵇOne death, caused by hemorrhagic stroke due to ruptured aneurysm. ᶜOne sudden CV death. ᵈOne CV death. ᵉOne death, caused by metastatic malignant melanoma. AE, adverse event; AESI, adverse event of special interest; csDMARD, conventional synthetic DMARD; CV, cardiovascular; GC, glucocorticoid; HZ, herpes zoster; MACE, major cardiovascular event; PBO, placebo; QD, once daily; UPA, upadacitinib; VTE, venous thromboembolic event.
ᵃIncludes non-treatment-emergent deaths. ᵇOne death, caused by hemorrhagic stroke due to ruptured aneurysm. ᶜOne sudden CV death. ᵈOne CV death. ᵉOne death, caused by metastatic malignant melanoma. AE, adverse event; AESI, adverse event of special interest; csDMARD, conventional synthetic DMARD; CV, cardiovascular; GC, glucocorticoid; HZ, herpes zoster; MACE, major cardiovascular event; PBO, placebo; QD, once daily; UPA, upadacitinib; VTE, venous thromboembolic event.
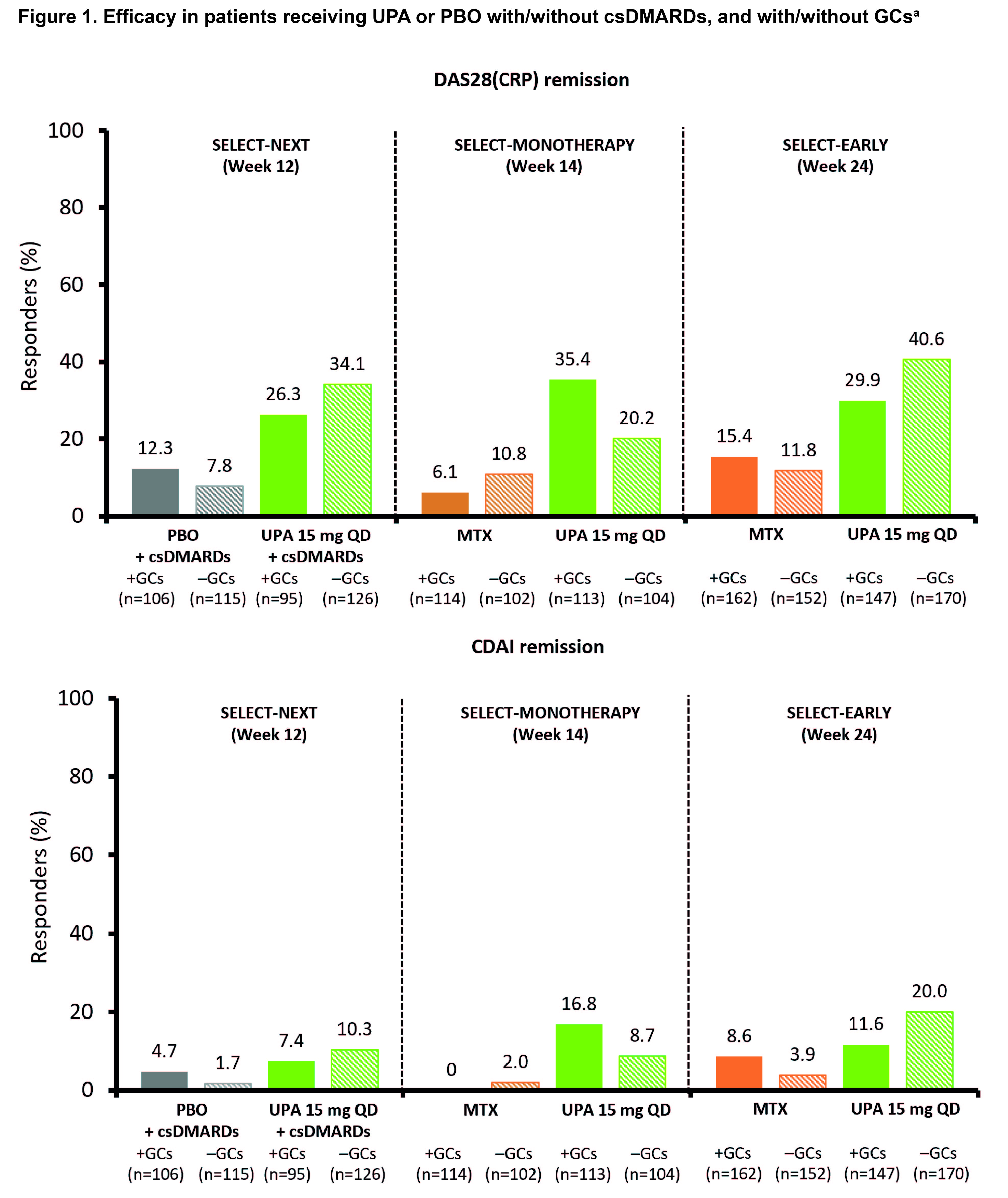 ᵃNon-responder imputation. CDAI, Clinical Disease Activity Index; csDMARD, conventional synthetic DMARD; DAS28(CRP), Disease Activity Score in 28 joints using CRP; GC, glucocorticoid; PBO, placebo; QD, once daily; UPA, upadacitinib.
ᵃNon-responder imputation. CDAI, Clinical Disease Activity Index; csDMARD, conventional synthetic DMARD; DAS28(CRP), Disease Activity Score in 28 joints using CRP; GC, glucocorticoid; PBO, placebo; QD, once daily; UPA, upadacitinib.
To cite this abstract in AMA style:
Combe B, Buttgereit F, Östör A, Xavier R, Saraux A, Daridon C, Famulla K, Song Y, Lagunes-Galindo I, Burmester G. Impact of Concomitant Glucocorticoids on the Clinical Efficacy and Safety of Upadacitinib in Patients with Rheumatoid Arthritis: An Ad Hoc Analysis of Data from Three Phase 3 Studies [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/impact-of-concomitant-glucocorticoids-on-the-clinical-efficacy-and-safety-of-upadacitinib-in-patients-with-rheumatoid-arthritis-an-ad-hoc-analysis-of-data-from-three-phase-3-studies/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-concomitant-glucocorticoids-on-the-clinical-efficacy-and-safety-of-upadacitinib-in-patients-with-rheumatoid-arthritis-an-ad-hoc-analysis-of-data-from-three-phase-3-studies/
