Session Information
Date: Sunday, November 8, 2020
Title: RA – Treatments Poster III: PROs, Biomarkers, Systemic Inflammation & Radiographs
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: In the analysis of PLANETRA, a Phase III randomized controlled trial (RCT), no significant association was found between body mass index (BMI) and clinical responses in rheumatoid arthritis (RA) patients using weight-based intravenous (IV) infliximab (3 mg/kg every 8 weeks)1,2. Since the subcutaneous (SC) formulation of CT-P13 which was approved from EMA after demonstrating non-inferiority compared to CT-P13 IV is a fixed-dose of 120 mg biweekly, it is meaningful to evaluate the impact of BMI on the clinical response of CT-P13 SC3,4. This study is to investigate the impact of BMI on clinical responses of CT-P13 SC 120 mg in Part 2 of Phase I/III RCT in active RA patients throughout the 1-year treatment period.
Methods: A total of 165 patients who received at least one full dose of CT-P13 SC (after IV infusions at Weeks 0 and 2 in the IV dose-loading phase) before Week 30 and who had at least one efficacy evaluation result after Week 6 or thereafter were included in this analysis. Patients were categorized into the three groups; under/normal weight (< 25kg/m2), overweight (≥25kg/m2, < 30kg/m2), and obesity (≥30kg/m2) based on the WHO BMI classification. Baseline characteristics, mean change from baseline in disease activity by DAS28 (CRP), remission (DAS28 [CRP]≤2.6) and low disease activity (LDA; 2.6< DAS28 [CRP]≤3.2), duration (week) of LDA, EULAR response, and ACR response were evaluated among the three BMI groups.
Results: In the under/normal weight (n=63), overweight (n=61), and obesity (n=41) groups, the mean±SD of age (years) (48.7±14.00, 51.5±10.93, 53.8±10.62) and the mean±SD of RA duration (years) (6.4±6.00, 8.2±9.27, 5.4±4.72) were comparable. At baseline, the rates of high disease activity (90.5%, 90.2%, 87.8%; DAS28 [CRP] >5.1) were similar among the groups. All other baselines and disease characteristics including gender, race, and other disease activity were also comparable among the groups. The rate of remission or LDA (Figure 1), the mean change from baseline of DAS28 (CRP) (-3.3, -3.1, -3.3 at Week 54), duration of LDA up to Week 54 (26.2, 29.2, 27.9 weeks), and the good or moderate EULAR responder rates (84.1%, 80.3%, 90.2% at Week 54) were all comparable among the groups and there were no statistically significant differences (p-value >0.05). The ACR responder rates were also comparable among the groups except for the ACR70 at Weeks 2 and 6 which were obtained following 2 IV infusions in the IV dose-loading phase. The absolute value of Pearson correlation coefficient was below 0.06 between BMI at baseline and mean change from baseline in DAS28 (CRP) during the CT-P13 SC treatment from Weeks 14 to 54, indicating that correlation between two measurements was weak and was not statistically significant (p-value >0.05) (Figure 2).
Conclusion: These results showed that there was no impact of BMI on the clinical responses of CT-P13 SC 120 mg biweekly in RA patients. Therefore, CT-P13 SC 120 mg is considered to be a valid therapeutic option regardless of BMI.
References:
- Yoo D.H., et al. Ann Rheum Dis, 2013;72:1613-20.
- Yoo D.H., et al. Ann Rheum Dis, 2016;75:1005.
- 3. Westhovens R., et al. Ann Rheum Dis, 2019;78:A1158
- Westhovens R., et al. Arthritis Rheumatol. 2019;71 (suppl 10).
 Figure 1. Remission or LDA Based on DAS28 (CRP)
Figure 1. Remission or LDA Based on DAS28 (CRP)
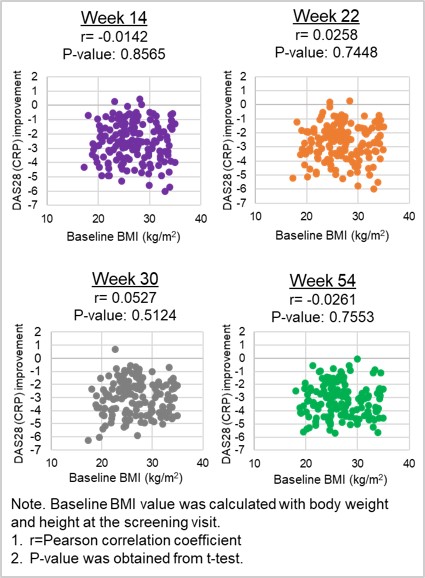 Figure 2 Correlation between BMI at Baseline and Mean Change from Baseline of DAS28 (CRP)
Figure 2 Correlation between BMI at Baseline and Mean Change from Baseline of DAS28 (CRP)
To cite this abstract in AMA style:
Yoo D, Westhovens R, Wiland P, Zawadzki M, Ivanova D, Berrocal Kasay A, Chalouhi E, Balázs E, Lee S, Kim S, Han N, Jung Y. Impact of Body Mass Index on Clinical Responses of Novel Subcutaneous Infliximab (CT-P13 SC) in Patients with Active Rheumatoid Arthritis: 1-Year Results from a Part 2 of Phase I/III Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/impact-of-body-mass-index-on-clinical-responses-of-novel-subcutaneous-infliximab-ct-p13-sc-in-patients-with-active-rheumatoid-arthritis-1-year-results-from-a-part-2-of-phase-i-iii-randomized-contro/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-body-mass-index-on-clinical-responses-of-novel-subcutaneous-infliximab-ct-p13-sc-in-patients-with-active-rheumatoid-arthritis-1-year-results-from-a-part-2-of-phase-i-iii-randomized-contro/
