Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Joint replacement surgery patterns continue to change in patients with rheumatoid arthritis (RA), possibly reflecting the widespread adoption of disease modifying antirheumatic drugs, earlier intervention and better supportive care. In a study of a population based RA sample from Rochester, Minnesota, a decline in cumulative incidence of orthopedic surgery by decade of RA diagnosis was reported.(1) Previous analyses based on the Swedish Hip Arthroplasty Register demonstrated a decline in the proportion of total hip arthroplasties due to inflammatory joint disease from 5% during 1992–2002 to 2% in 2007.(2)
Methods: We performed a retrospective analysis of pharmacy and medical claims data from 2003–2010 in the United States utilizing the MarketScan database. Patients were required to be continuously enrolled preceding 1–3 years post RA diagnosis. We used a coding algorithm to identify all patients having a diagnosis of RA and no prior use of biologics ≤1 yr preceding the diagnosis. From this pool, we stratified patients who did and did not receive a biologic within 6 months, 1 , 2, and 3 yrs post diagnosis. We further coded the analyses to identify total hip or knee replacement events (THR/TKR) and mean days to THR/TKR in these sub-groups and estimated general population rates of THR/TKR.
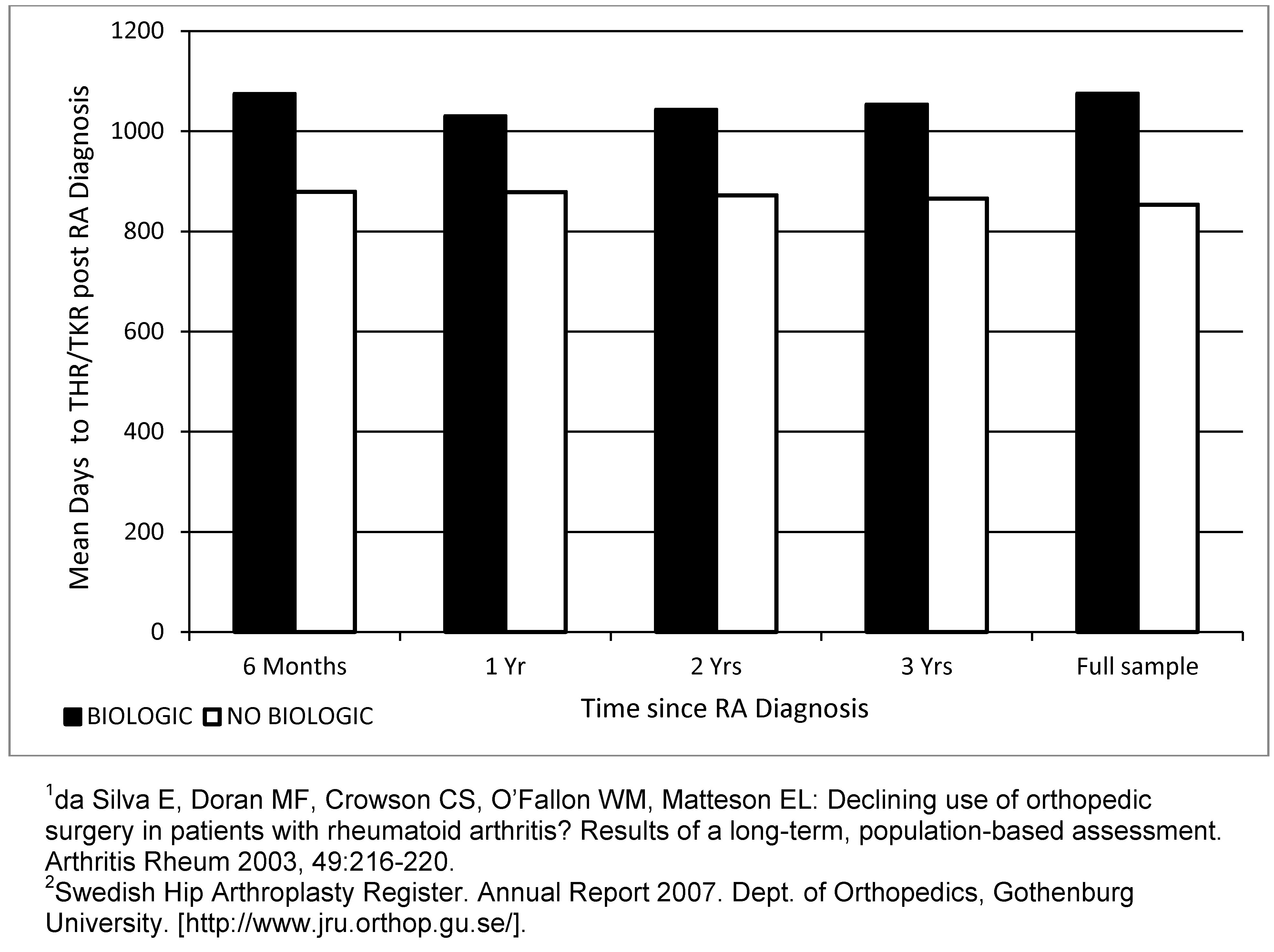
Results: From 2003–2010, 90,545 patients were identified with a diagnosis of RA; mean age 56.5 yrs; majority were female (71%) with low comorbidity [Charlson Comorbidity Index (CCI) 0.77]. Of these, 10,492 (11.5%) initiated a biologic and nearly half (5,054/10,492; 48%) received the biologic ≤1 yr of diagnosis. No differences in age and other demographic variables between groups at baseline; however the biologic cohort had higher proportions of rheumatologic disease in the CCI. Proportions of THR/TKR in the non-biologic cohort were ~7% vs. ~11% in the more severe biologic cohort over the follow-up period (p<0.0001). In spite of the higher event rate, patients in the biologic cohort were event free for an additional mean 221 days compared to the non-biologic cohort (p<0.0001). THR/TKR event rates in the general population in MarketScan were 0.49% (484,107/98,414,730) compared to 6.91% (10,927/158,187) in the RA specific cohort (regardless of treatment).
Conclusion: The rates of THR/TKR were slightly higher in the supposedly more severe RA population who initiated a biologic (CCI 0.88), as opposed to those who did not (CCI 0.76). The mean time to THR/TKR was significantly delayed in the biologic cohort by up to 7 months, despite the higher proportion of rheumatologic disease in CCI. These results are compatible with the extensive evidence for delayed peripheral joint damage in RA patients treated with TNF inhibitors. Results must be interpreted by acknowledging the non-randomized nature of the claims data and inability to adjust for RA severity at baseline due to lack of relevant database variables.
Disclosure:
A. S. Koenig,
Pfizer Inc,
3,
Pfizer Inc,
1;
J. Mardekian,
Pfizer Inc,
3,
Pfizer Inc,
1;
S. Kotak,
Pfizer Inc,
1,
Pfizer Inc,
3.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-biologics-on-total-knee-replacement-and-total-hip-replacement-rates-in-rheumatoid-arthritis-patients-results-from-us-marketscan-database/