Session Information
Date: Sunday, November 5, 2017
Title: Systemic Lupus Erythematosus – Clinical Aspects and Treatment Poster I: Biomarkers and Outcomes
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: There is a lack of phenotypic data on SLE patients who have never been on immunosuppressive therapy. Anti-RNA binding protein (anti-RBP) antibodies (anti-Smith, anti-RNP, anti-SSA, anti-SSB) in SLE have been linked to an upregulation in transcriptional patterns, some thought to contribute to disease. This study sought to characterize the impact of these autoantibodies on disease activity and quality-of-life in immunosuppressant naïve SLE patients.
Methods: Retrospective cross-sectional analysis of subjects meeting ACR Classification Criteria for SLE was performed. Subjects were anti-RBP positive (RBP+) if at least one titer was ≥ 20 (units, ELISA); others were classified as anti-RBP negative (RBP-). Subjects ever on non-corticosteroid immunosuppression or currently on corticosteroids were excluded. Baseline characteristics included age, sex, disease duration, anti-dsDNA, C3, C4, and ethnicity (African-American [AA] or European-American [EA]). A measure of all four anti-RBP titers and most baseline characteristics were necessary for inclusion. Disease activity was measured using the SLEDAI and quality-of-life indices included the Beck Depression Inventory (BDI), Fatigue Severity Scale (FSS), Numerical Rating Scale for Pain (NRS-Pain Scale), and the Medical Outcomes Study Sleep Scale (MOS-SS). Both groups were compared using Mann-Whitney U, two-tailed Fisher’s Exact tests, and multivariate linear regression.
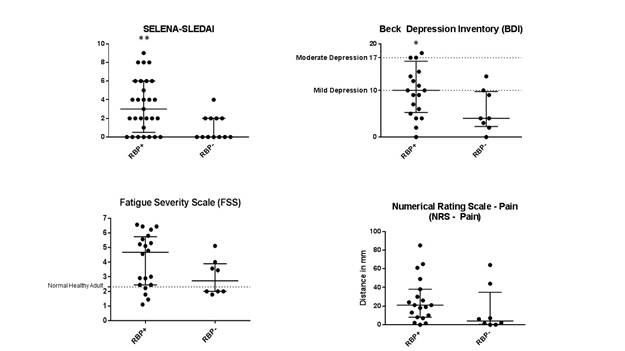
Results: Forty-one subjects met criteria for inclusion, 29 of which were identified as RBP+ and 12 RBP-. There was no difference in age, sex, disease duration, anti-dsDNA, C3, or C4 between groups. There were significantly more AA in the RBP+ group (65.5% vs 16.7%, p <0.01) and EA in the RBP- group (34.5% vs 75.0%, p=0.037). Disease activity and quality-of-life indices between groups is demonstrated in Figure 1. SLEDAI was significantly worse in RBP+ vs RBP- subjects (3 vs. 0, p=0.009), a relationship confirmed with multivariate regression (standardized beta coefficient = 0.515, p = 0.017). Similarly, BDI scores were worse in the RBP+ cohort (10 vs. 4, p=0.050). RBP+ subjects trended towards worse fatigue by FSS (4.67 vs. 2.72, p=0.139) and pain by NRS-Pain Scale (21 vs. 4, p=0.087). MOS-SS sleep indices were also worse among RBP+ subjects. To account for the difference in ethnicity between groups, a subgroup analysis of EA patients was conducted and found similar trends in RBP+ patients.
Conclusion: Anti-RBP positive SLE subjects showed worse measures of disease activity and quality-of-life. RBP+ subjects were more likely to be African-American, although similar outcomes were seen in a European-American sub-analysis. Our study was limited by sample size given our exclusion of immunosuppressed subjects. Further study is needed to measure these antibodies’ impact on clinical outcomes and response to treatment prospectively.
To cite this abstract in AMA style:
Bermea R, Utset T, Ko K. Impact of Anti-RBP Antibodies on Disease Activity and Quality-of-Life in Immunosuppressant Naive Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/impact-of-anti-rbp-antibodies-on-disease-activity-and-quality-of-life-in-immunosuppressant-naive-systemic-lupus-erythematosus/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-anti-rbp-antibodies-on-disease-activity-and-quality-of-life-in-immunosuppressant-naive-systemic-lupus-erythematosus/