Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Ankylosing spondylitis (AS), a chronic inflammatory disease involving the sacroiliac joints and spine, is associated with pain, stiffness, disability, and reduced quality of life.1,2 Therefore, prompt diagnosis and treatment of patients (pts) is critical. In this post hoc analysis, we assessed the impact of age and disease duration (DD; defined as time since diagnosis) on responses in pts with AS treated with secukinumab (SEC) or placebo (PBO) in the phase 3 MEASURE trials.
Methods: Pts with active AS from MEASURE 1 (M1; NCT01358175), 2 (M2; NCT01649375), 3 (M3; NCT02008916), and 4 (M4; NCT02159053) randomized controlled trials (RCTs) were included.3-8 Pts received subcutaneous (SC) SEC 300 or 150 mg with an intravenous (IV) loading dose (M1, M3), SEC 150 mg with an SC loading dose (M2, M4), or PBO (M1-4). Pooled data from different RCTs (IV or SC loading) may be considered heterogeneous and are generalizable to a wider patient population. Data were pooled from M1-4 at the end of the 16-wk treatment period, and responses were analyzed according to 4 age groups: 18-33, 34-42, 43-51, and 52-79 y. Responses were assessed using outcome measures such as ASAS20/40, BASDAI, BASMI, hsCRP, SF-36 (PCS/MCS), and disease activity/back pain instruments. Similar analyses assessed treatment responses by quartiles of time since diagnosis (0-0.97 y [Q1], 0.98-3.47 y [Q2], 3.49-9.79 y [Q3], and 9.88-50.19 y [Q4]). Time since diagnosis was used as a surrogate marker of DD as symptom duration was not collected. No adjustment was made for multiple comparisons.
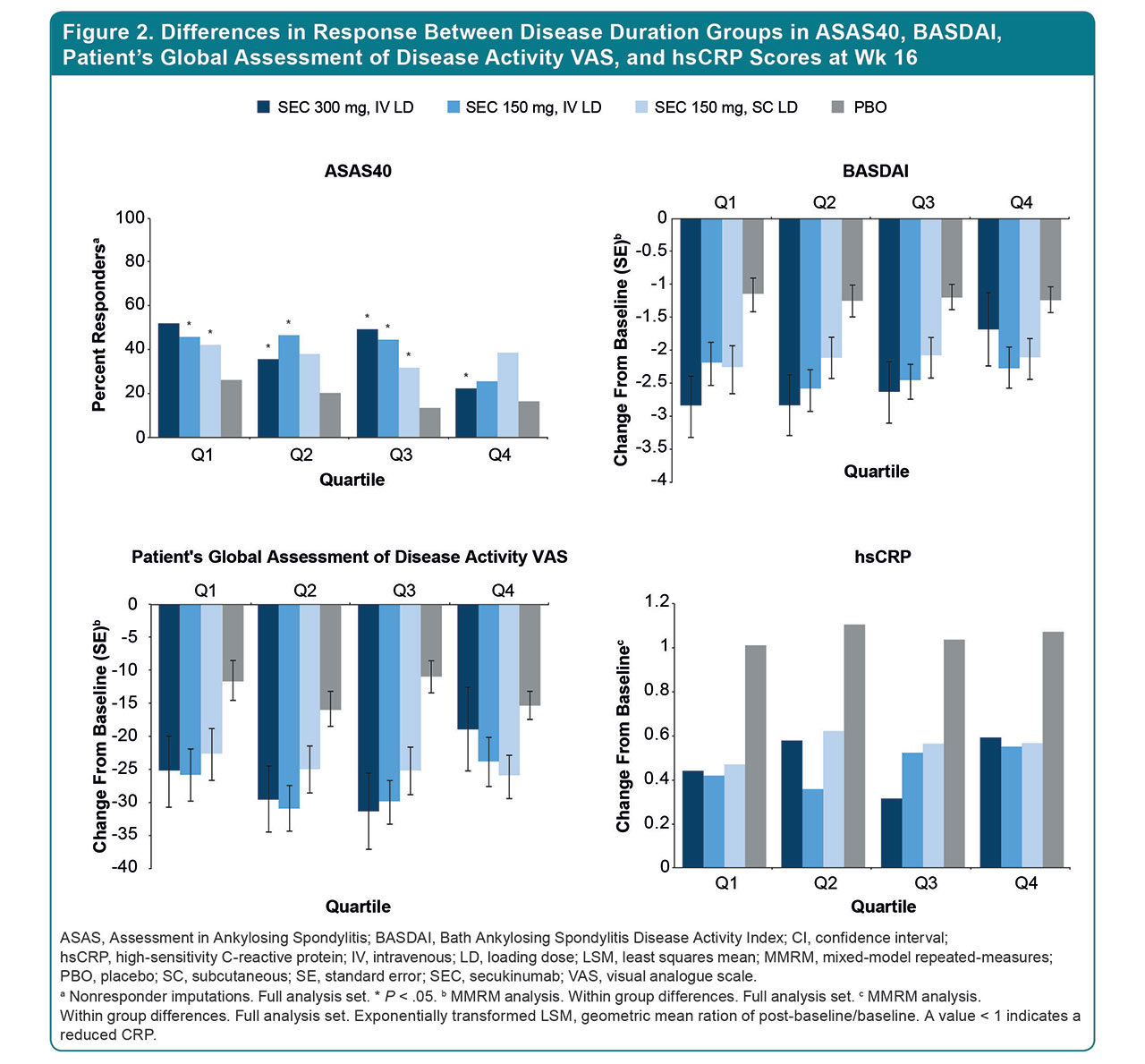
Results: 852 pts were included in this analysis (SEC, n = 463; PBO, n = 389) (Table 1); 95.5% completed 16 wk of treatment. Baseline demographics were similar across trials (Table 1). Mean time since AS diagnosis differed across age groups: 3.2 y in the 18-33 group and 11.3 y in the 52-79 group. The proportion of TNF inhibitor–naive pts was higher in the 18-33 group vs the 52-79 group (74.4% vs 65.0%). At Wk 16, greater improvements in ASAS20/40, BASDAI, SF-36, hsCRP, and VAS disease activity/back pain scores were reported in younger (18-33 and 34-42) vs older age groups (43-51 and 52-79) treated with SEC (Fig. 1). When stratified by DD, there was a higher proportion of TNF inhibitor–naive pts in Q1 vs Q4 (92.0% vs 61.8%), and pts with a shorter DD (Q1-Q2) had greater improvements in ASAS40; reductions in hsCRP levels were greatest in Q1 pts (Fig. 2).
Conclusion: SEC treatment led to rapid and sustained improvements in all outcome measures at Wk 16, regardless of age or DD. Older pts reported greater burden of disease. A trend toward higher responses was evident in those with shorter DD. Younger pts had better responses, likely due to shorter DD and a higher proportion being biologic naive. These results emphasize the importance of early AS treatment to delay disease progression and improve pt outcomes.
References:
- Braun J, Sieper J. Lancet. 2007;369:1379-1390.
- Bodur H, et al. Qual Life Res. 2011;20:543-549.
- Baeten D, et al. N Engl J Med. 2015;373:2534-2548.
- Wei JC, et al. Int J Rheum Dis. 2017;20:589-596.
- Baraliakos X, et al. Clin Exp Rheumatol. 2018;36:50-55.
- Marzo-Ortega H, et al. RMD Open. 2017;3:e000592.
- Pavelka K, et al. Arthritis Res Ther. 2017;19:285.
- Kivitz AJ, et al. Rheumatol Ther. 2018;5:447-462.
To cite this abstract in AMA style:
Deodhar A, Mease P, Machado P, Meng X, Strand V, Magrey M. Impact of Age and Disease Duration on the Response to IL-17A Inhibitor (Secukinumab) Treatment in Ankylosing Spondylitis: Pooled Results from the Phase 3 MEASURE Studies [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/impact-of-age-and-disease-duration-on-the-response-to-il-17a-inhibitor-secukinumab-treatment-in-ankylosing-spondylitis-pooled-results-from-the-phase-3-measure-studies/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-age-and-disease-duration-on-the-response-to-il-17a-inhibitor-secukinumab-treatment-in-ankylosing-spondylitis-pooled-results-from-the-phase-3-measure-studies/