Session Information
Date: Tuesday, November 9, 2021
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes (1909–1914)
Session Type: Abstract Session
Session Time: 3:15PM-3:30PM
Background/Purpose: Due to concerns about underlying immune dysregulation and immunosuppression, patients with systemic rheumatic diseases (SRD) may have modified their medications at the time of COVID-19 vaccination to optimize their immune response. Although the American College of Rheumatology COVID-19 Vaccine Clinical Guidance Task Force issued management guidelines on February 8, 2021, it is unclear how rapidly these guidelines were disseminated. Our study evaluated real-world medication modification at the time of COVID-19 vaccination by Rheumatology patients at a single center in New York City.
Methods: We emailed a secure web-based survey to 7,505 patients aged ≥18 years evaluated at least once by a rheumatologist between April 1, 2018-April 21, 2020 at a large Rheumatology center in New York City. A follow-up survey was sent between March 5, 2021 and March 25, 2021. In the current analysis, we included individuals who indicated receiving at least the 1st vaccine dose. We collected data on immunomodulatory and immunosuppressive medication usage and modifications at the time of COVID-19 vaccination. We also collected the source responsible for the medication modifications (i.e. rheumatologist, other physician, patient).
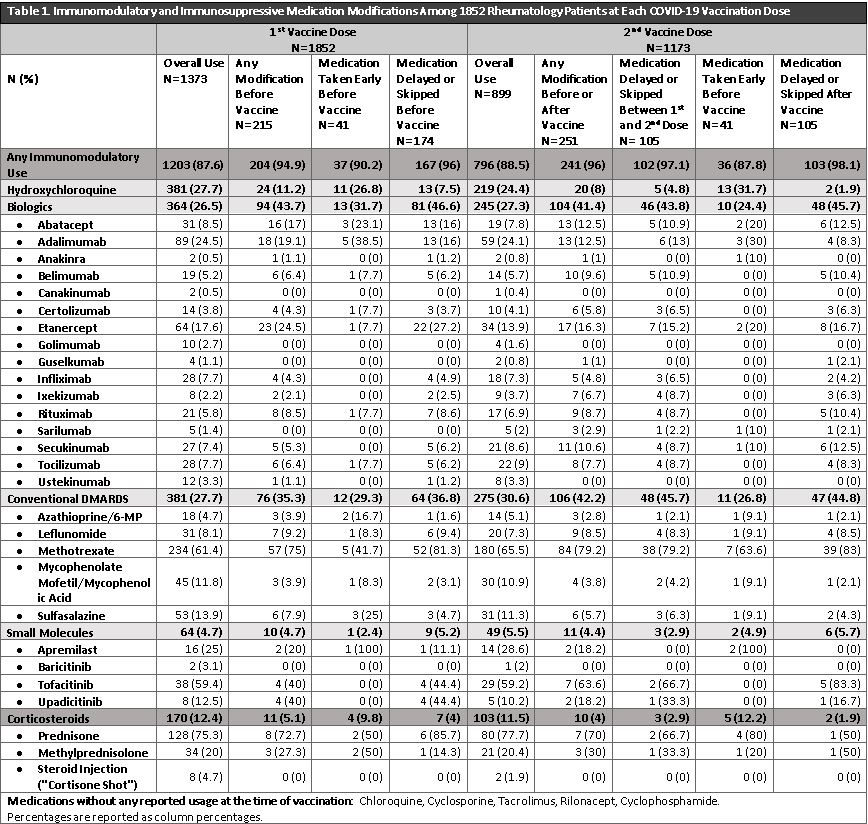
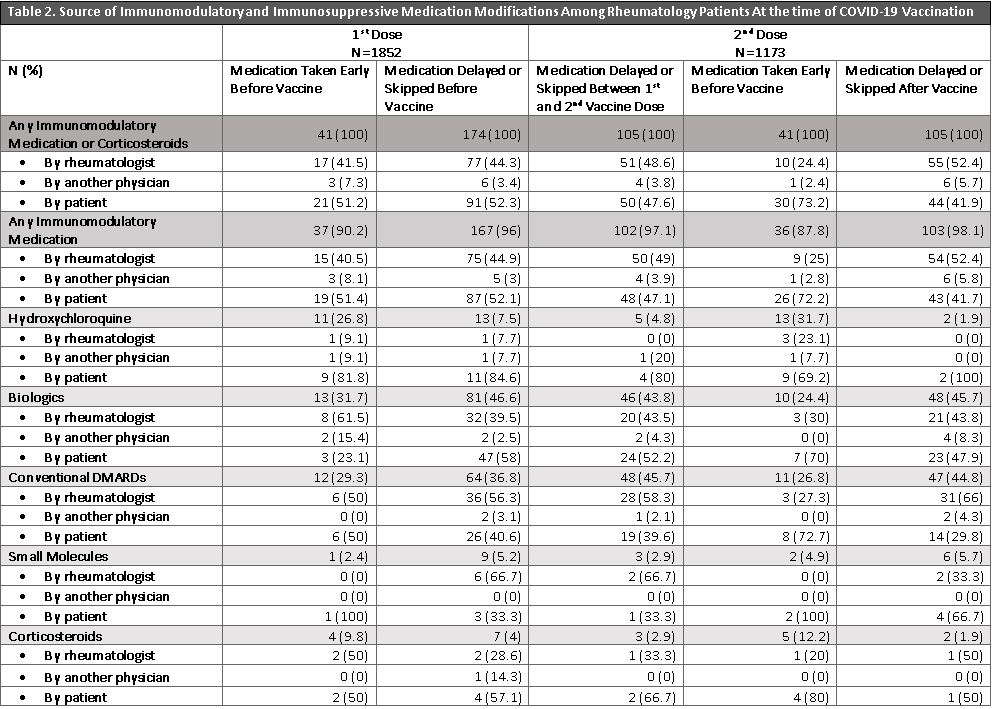
Results: 1852/7505 (24.7%) patients responded to vaccination and medication modification questions (mean age: 63±13.2 years, 79.6% female, 87.4% white, 4.8% Hispanic/Latinx). 1852 patients received at least 1COVID-19 vaccine dose (53.9% Pfizer, 44.3% Moderna, 1.4%% Janssen, 0.38% Other) and 1173 reported having two doses. At 1st dose, there were 1373 individual reports of immunomodulatory or corticosteroid use (27.7% hydroxychloroquine, 26.5% biologics, 27.7% conventional DMARDs, 12.7% corticosteroids, 4.7% small molecules, 0.9% other DMARDs). Before the 1st vaccine dose, 204 medications (15.7%) were modified; of these, 19% were taken earlier than scheduled, and 81% were delayed/skipped. At 2nd dose, out of 899 individual medication reports, 251 medications (27.9%) were modified. Of these, 105 medications (41.8%) were delayed/skipped between the 1st and 2nd dose, 41 (16.3%) were taken earlier than scheduled and 105 (41.8%) were delayed/skipped after the 2nd vaccine dose. Biologics, typically anti-TNF inhibitors, and conventional DMARDs, typically methotrexate, accounted for most of the medications modified before or after the vaccine doses (Table 1). Patients modified their own medications >50% of the time before the 1st vaccine dose, and opted to take medication early >70% of the time before the 2nd vaccine dose (Table 2). Over half of the medications that were delayed/skipped after the 2nd vaccine were recommended by a rheumatologist (Table 2).
Conclusion: Up to 28% of immunomodulatory or immunosuppressive medications were modified around the time of COVID-19 vaccination, and patients were responsible for up to 73% of modifications. The wide variety of medication modification provides insight into patient behavior and underscores the need for increased usage of evidence-based guidelines to inform patient and physician guidelines. Future studies will assess the implications of these medication modifications on SRD activity and flares post-vaccination.
To cite this abstract in AMA style:
Levine J, Jannat-Khah D, Bykerk V, Mandl L, Barbhaiya M. Immunomodulatory and Immunosuppressive Medication Modification Among Rheumatology Patients at the Time of COVID-19 Vaccination [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/immunomodulatory-and-immunosuppressive-medication-modification-among-rheumatology-patients-at-the-time-of-covid-19-vaccination/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunomodulatory-and-immunosuppressive-medication-modification-among-rheumatology-patients-at-the-time-of-covid-19-vaccination/