Session Information
Date: Monday, November 13, 2023
Title: (1264–1307) RA – Diagnosis, Manifestations, and Outcomes Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: In August 2021, the CDC recommended a third SARS-CoV-2 mRNA vaccine dose to complete the initial vaccine series for immunosuppressed patients who had previously received 2 mRNA doses. Despite this, some rheumatoid arthritis (RA) patients may remain at increased risk for breakthrough COVID-19 infection, due to use of immunomodulators which lead to blunted immune responses to vaccination and infection. Identifying patients at highest risk for breakthrough infection despite 3 vaccines can prioritize resources for prevention. Therefore, we investigated the risk of breakthrough COVID-19 among RA patients with at least 3 vaccine doses using disease-modifying antirheumatic drugs (DMARDs), especially TNF inhibitors (TNFi) and CD20 inhibitors (CD20i), during the Omicron wave.
Methods: We performed a retrospective cohort study investigating DMARDs and risk for breakthrough COVID-19 among RA patients who had received 3 mRNA vaccines at a large health care system. A previously validated algorithm was used to identify RA patients using a combination of diagnosis codes and DMARD/glucocorticoid prescription (PPV 90%). COVID-19 was identified systematically from positive PCR tests or patient reports to the hospital/clinic of home rapid antigen tests. Patients were followed from the date of 3rd vaccine (index date) until breakthrough COVID-19, non-COVID death, or end of follow-up (January 18, 2023). A previously defined hierarchical algorithm was used to categorize DMARD exposures. Covariates included demographics, lifestyle, comorbidities, and prior COVID-19. We used Cox proportional hazards models to estimate the risk of COVID-19 among different DMARD users. We then used propensity scores and overlap-weighting to further account for confounding when comparing the risk among users of CD20i vs. TNFi.
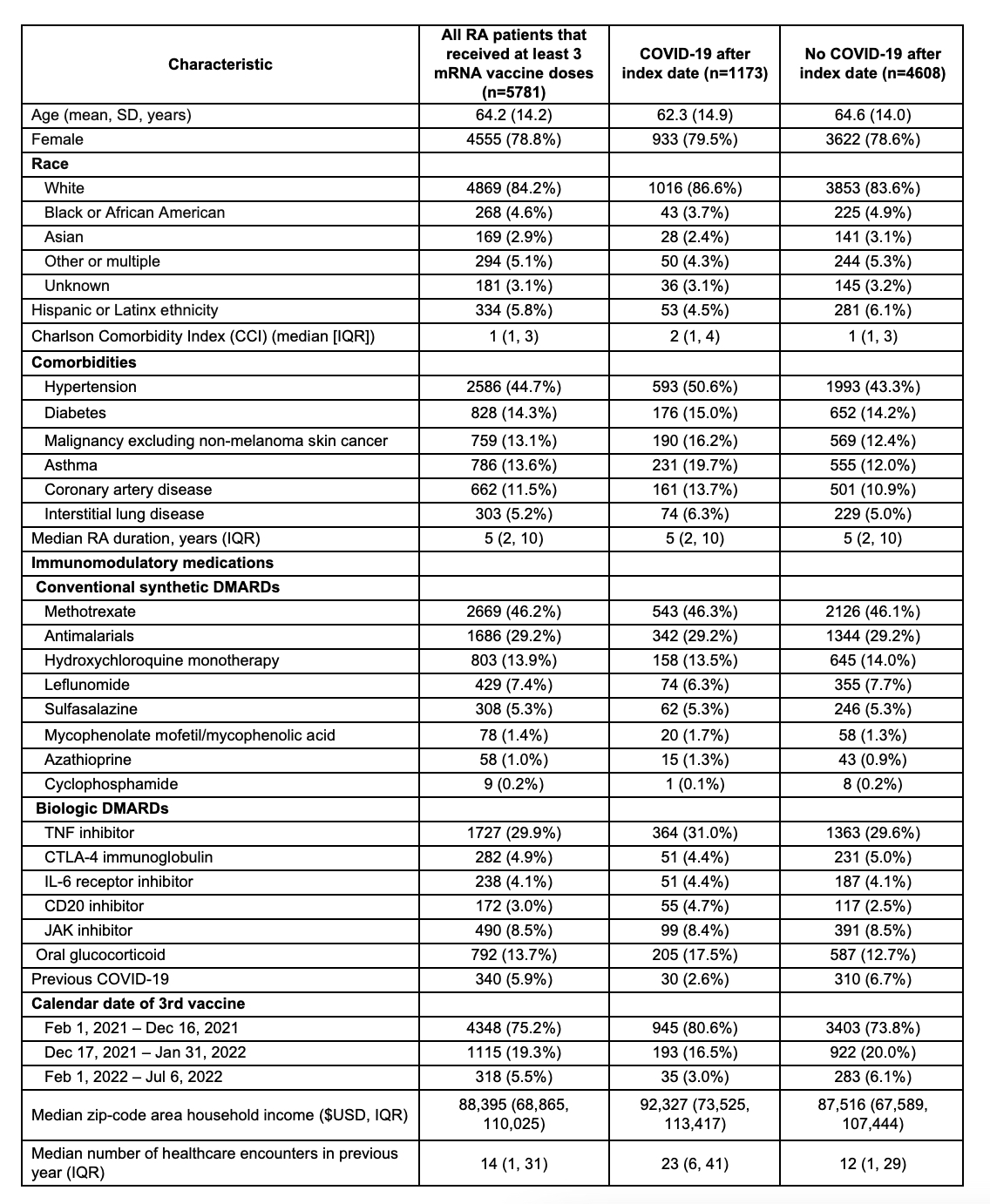
Results: We identified 5781 patients with RA who used DMARDs/glucocorticoids and received 3 mRNA vaccine doses (78.8% female, mean age 64.2 years). During mean follow up of 12.8 months, 1173 (20.2%) had a SARS-CoV-2 infection (incidence rate 15.7 per 1000 person-months) (Table 1). Users of CD20i were more likely than TNFi users to have COVID-19 after the index date (adjusted HR 1.74, 95%CI 1.30-2.33), as were users of steroid monotherapy (adjusted HR 1.47, 95%CI 1.09-1.98) (Figure 1). After using propensity scores for overlap weighting to account for baseline differences, CD20i users remained at higher risk for COVID-19 than TNFi users (HR 1.62, 95%CI 1.02-2.56; Figure 2). In a sensitivity analysis excluding patients with cancer or interstitial lung disease, the findings were similar.
Conclusion: In this RA-specific study evaluating the impact of DMARD use on the risk of COVID-19 during the Omicron phase of the pandemic, we identified CD20i and steroid monotherapy use as risk factors for breakthrough COVID-19. This contemporary study highlights the excess risk associated with glucocorticoid monotherapy or CD20 inhibitor use for RA treatment and identifies important subgroups for counseling and additional risk mitigation strategies.
To cite this abstract in AMA style:
Schiff A, Wang X, Patel N, Kawano Y, Hanberg J, Kowalski E, Cook C, Vanni K, Qian G, Bade K, Saavedra A, Srivatsan S, Williams Z, Venkat R, Wallace Z, Sparks J. Immunomodulators and Risk for Breakthrough COVID-19 After a Third SARS-CoV-2 mRNA Vaccine Among Patients with Rheumatoid Arthritis: A Cohort Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/immunomodulators-and-risk-for-breakthrough-covid-19-after-a-third-sars-cov-2-mrna-vaccine-among-patients-with-rheumatoid-arthritis-a-cohort-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunomodulators-and-risk-for-breakthrough-covid-19-after-a-third-sars-cov-2-mrna-vaccine-among-patients-with-rheumatoid-arthritis-a-cohort-study/