Session Information
Date: Tuesday, November 15, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: A portion of patients without clinical response to anti-inflammatory biologics have been shown to develop anti-drug antibodies (ADA).1 ADA prevalence varies widely among biologics, with variability attributed to several factors, particularly use of a broad range of assay formats. A systematic literature review was conducted to specifically assess immunogenicity of biologics, differences in ADA assays, and associated clinical outcomes.
Methods: MEDLINE, EMBASE, and Cochrane databases, conference proceedings and review articles were searched for randomized controlled trials and observational studies of biologic therapies published before 4 September 2015 in which assay methods for ADA testing were reported. This analysis focuses on adalimumab and infliximab as they have been available for the longest period, with the greatest number of published studies and reports of immunogenicity.
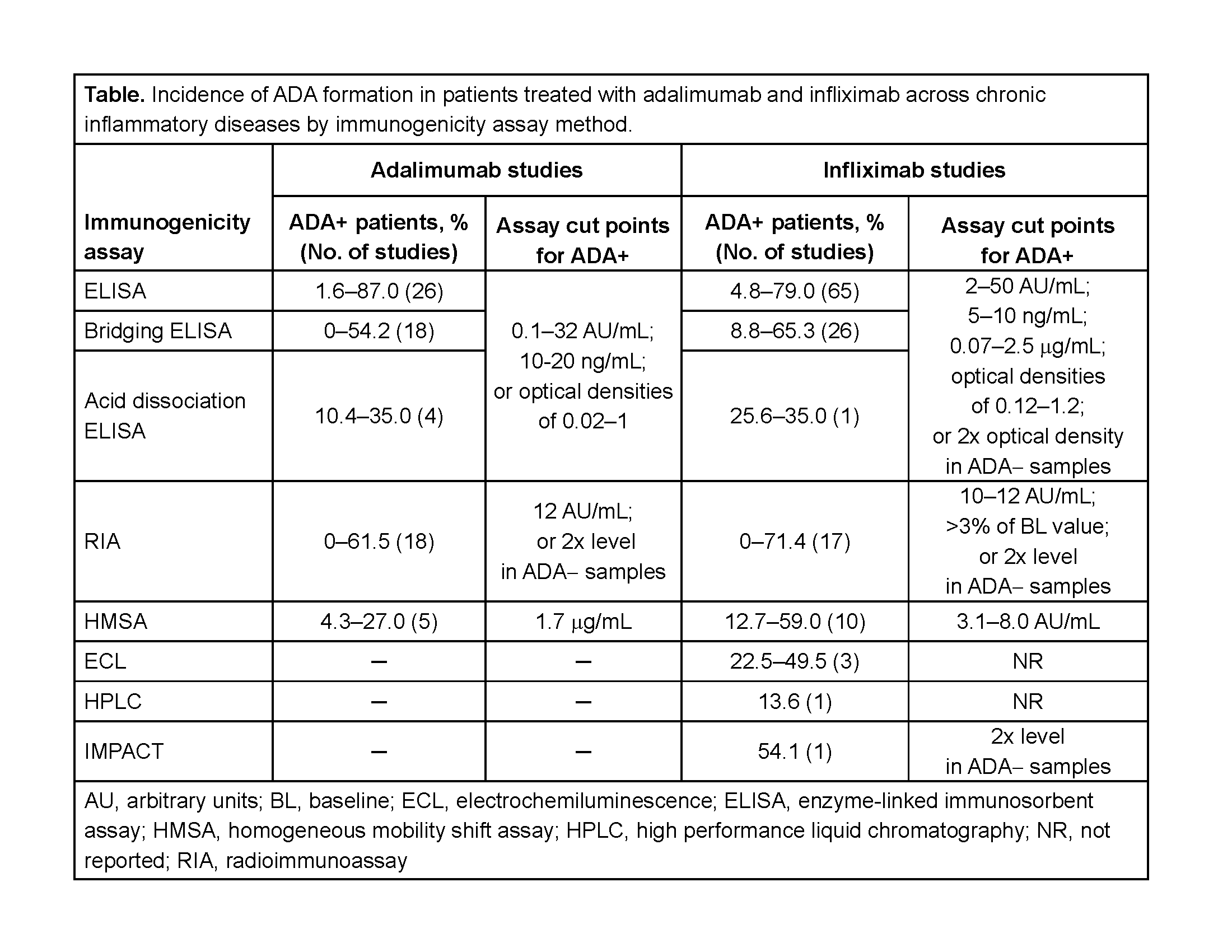
Results: Of 21,889 publications screened and 358 publications (322 studies) included, 71 and 123 studies of adalimumab and infliximab, respectively, were retained. In >20% of studies in which adalimumab and infliximab ADA were assessed, the assay method was not reported. In most adalimumab and infliximab studies, an ELISA (48/71 and 92/123) or radioimmunoassay (RIA; 17/71 and 16/123) was used (table). ADA+ incidence varied widely in studies of adalimumab and infliximab across inflammatory diseases (table). Clinical and pharmacokinetic outcomes were only reported for ADA+ patients in 39% and 45% of adalimumab and infliximab studies, respectively. ADA formation was consistently associated with lower serum concentrations of both biologics and elevated rates of infusion-related reactions with infliximab.
Conclusion: To improve interpretation of immunogenicity data for biologics, greater consistency is needed in reporting of assay methods as well as clinical consequences of ADA formation. Standardization in immunogenicity testing and reporting as recently proposed by Shankar et al2, application of modern assays with higher sensitivity and lower drug interference, and implementation of international standards for marketed products would allow greater insight into the impact of immunogenicity to biologics. References: 1. van Schouwenburg, PA et al. Nature Revs Rheum. 2013;9:164-72. 2. Shankar, G et al. AAPS J. 2014;16(4):658-73.
To cite this abstract in AMA style:
Gorovits B, Baltrukonis D, Bhattacharya I, Birchler MA, Finco D, Sikema D, Vincent MS, Lula S, Marshall L, Hickling T. Immunogenicity Assessment of Biologics in Clinical Studies in Chronic Inflammatory Disease: A Systematic Review [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/immunogenicity-assessment-of-biologics-in-clinical-studies-in-chronic-inflammatory-disease-a-systematic-review/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunogenicity-assessment-of-biologics-in-clinical-studies-in-chronic-inflammatory-disease-a-systematic-review/