Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: The VOLTAIRE trials program compared the safety, efficacy, and immunogenicity of biosimilar BI 695501 with adalimumab reference product (RP) for indications including moderate-severely active rheumatoid arthritis (RA), Crohn’s disease (CD), and chronic plaque psoriasis (PsO).1,2,3 These analyses compare immunogenicity across these indications and by patient sex.
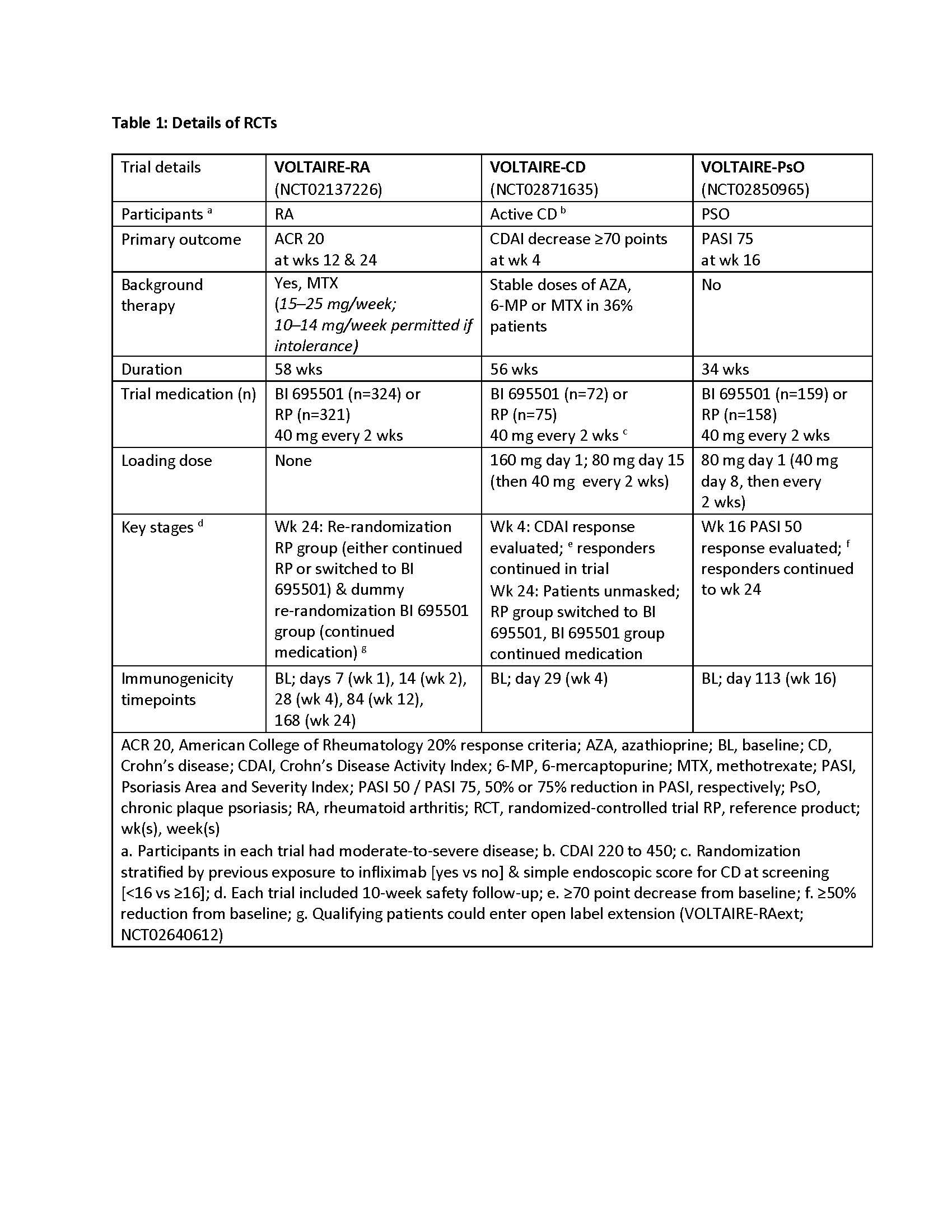
Methods: Details of each active-comparator, randomized controlled trial (RCT) are presented in Table 1. Immunogenicity was assessed at various timepoints by the proportion of patients with anti-drug antibodies (ADAs) and neutralizing antibodies (nAbs), using acid dissociation followed by an electrochemiluminescence assay (ECL; MSD platform; Meso Scale Diagnostics LLC, USA).4 Assay sensitivity was 50 ng/mL, and drug tolerance ≥30 μg/mL (free drug) at the low positive control level.
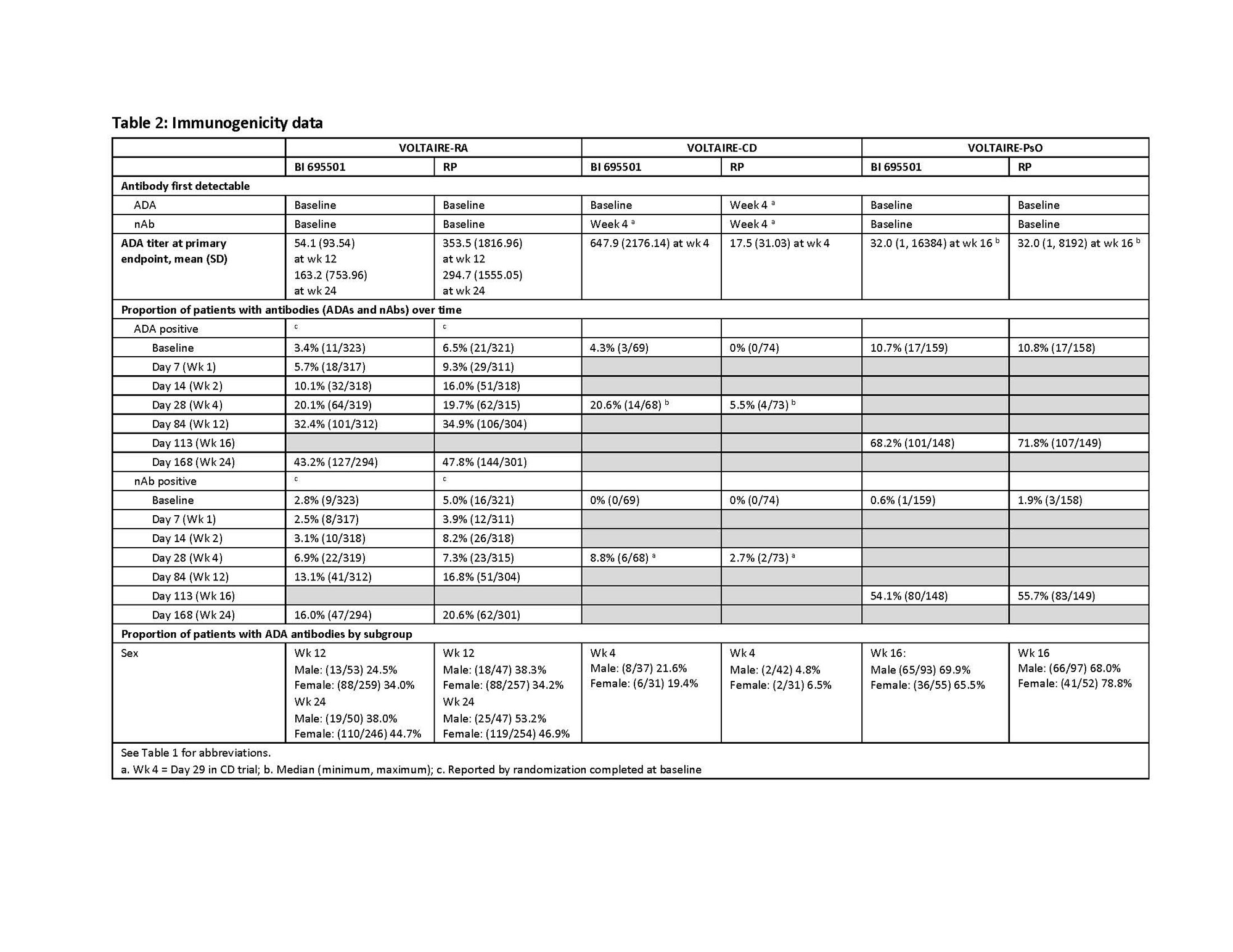
Results: Data are presented in Table 2.
Conclusion: These data demonstrate minor differences in immunogenicity parameters (ADAs, ADA titers and nAbs) between BI 695501 and adalimumab RP across these 3 immune-mediated inflammatory diseases (IMIDs). The proportion of ADA- and nAb-positive patients increased from baseline over time in all 3 RCTs, as expected, and were similar in the RA and CD RCTs. However, higher rates of ADA- and nAb-positive patients were reported in the PsO trial. Subgroup analysis by patient sex showed the same trend. Differences among the RCTs may be partially explained by concomitant background therapy (MTX) in the RA trial, stable doses of AZA, 6-MP or MTX in 36% of CD patients and the absence of background therapy in the PsO RCT. In addition, comparisons are limited by the different visit schedules among the trials. Historical comparisons to data for the RP are complicated by recent differences in regulatory requirements for increased ADA assay sensitivity and stringency for biosimilar products than those originally used for the RP. Acid dissociation followed by the more sensitive ECL assay for detection of ADAs is not dependent on serum drug concentrations. In conclusion, these analyses confirm the biosimilarity of BI 695501 with the adalimumab RP across IMIDs.
To cite this abstract in AMA style:
Strand V, Bender S, McCabe D. Immunogenicity Analysis from the VOLTAIRE Trials in Patients with Rheumatoid Arthritis, Crohn’s Disease, and Chronic Plaque Psoriasis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/immunogenicity-analysis-from-the-voltaire-trials-in-patients-with-rheumatoid-arthritis-crohns-disease-and-chronic-plaque-psoriasis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunogenicity-analysis-from-the-voltaire-trials-in-patients-with-rheumatoid-arthritis-crohns-disease-and-chronic-plaque-psoriasis/