Session Information
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: Patients with immune-mediated inflammatory diseases (IMIDs) are at increased risk of severe COVID-19 disease courses. Real-world data on immunogenicity are scarce but crucial to develop vaccination strategies to increase the level of protection in such patients at risk. We aimed to investigate the humoral immune response to primary vaccination against SARS-CoV-2 in IMID patients as compared to healthy controls (HCs), considering the effect of disease and immunosuppressive therapy.
Methods: In this retrospective, monocentric cohort study adult ( >18 years) IMID patients, who received a primary COVID-19 vaccination, were included. Patients receiving rituximab, reported history of SARS-CoV-2 infection or antibodies against nucleocapsid were excluded. Data on disease entity, immunosuppressive therapy including steroids, and information on date and compound of SARS-CoV-2 vaccination were obtained using electronic medical records. Quantification of antibodies to the receptor-binding domain (RBD) of the viral spike (S) protein (range between 0.4 and 2500.0 binding antibody units [BAU/mL]) was performed. Individuals from an ongoing prospective population-based study were included as HC. Difference in antibody levels between groups are shown as median and inter quartile range (IQR). To assess the role of the individual disease entities and immunosuppression in the anti-RBD antibody response (dependent variable), we used a multivariable linear regression model with robust standard errors to account for heteroscedasticity. The covariates of the model were selected based on previous clinical research: age (years), gender (male/female), vaccination strategy (BNT162b2, mRNA-1273, ChAdOx1 nCoV-19 or others), time since primary vaccination and concomitant corticosteroid treatment.
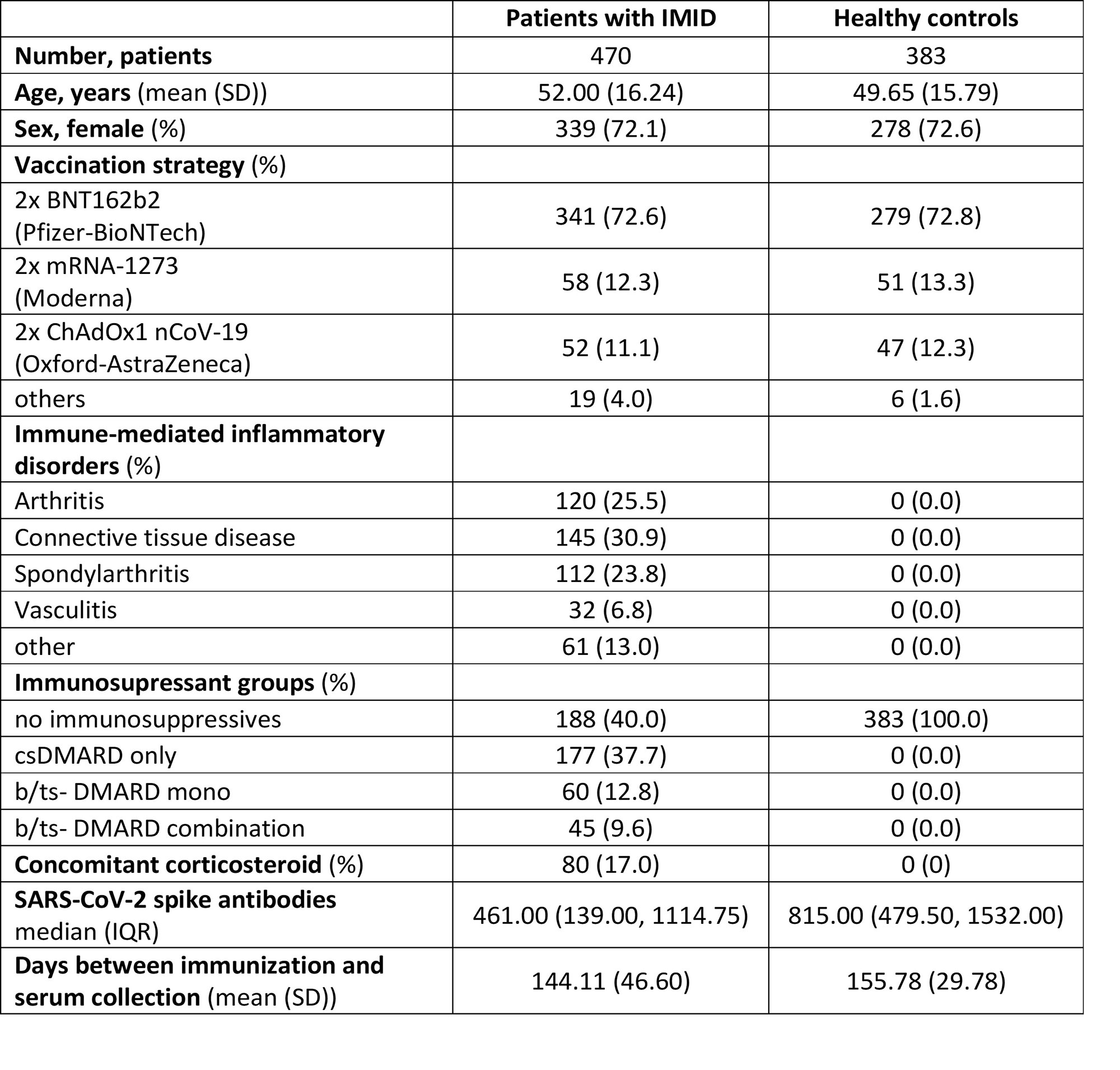
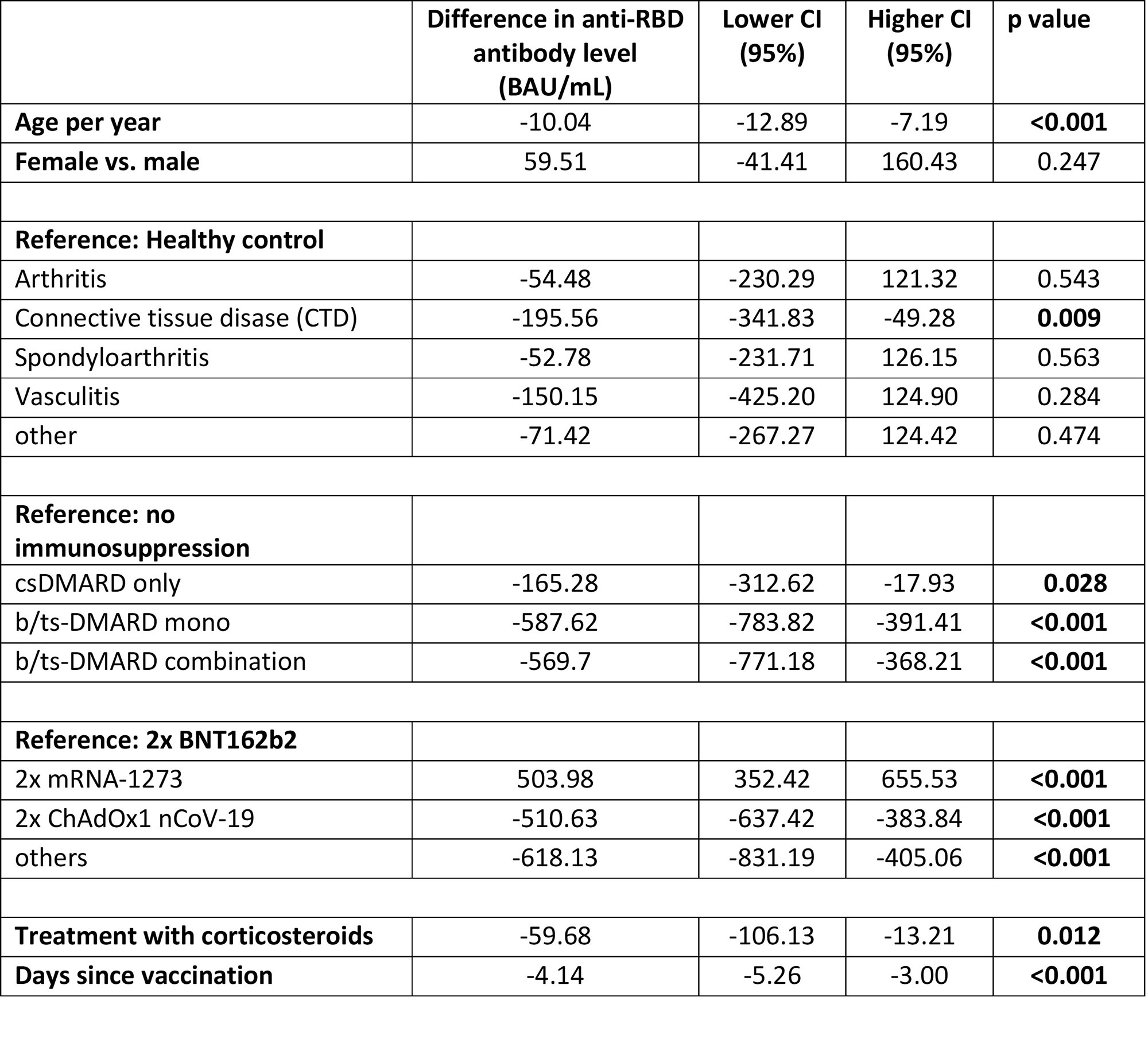
Results: 470 patients with IMIDs and 383 HCs were included for analyzes (for population characteristics see table 1). Overall, median levels [IQR] of antibodies were higher in individuals vaccinated with mRNA-1273 (867 BAU/ml [240, 2452]) compared to BNT162b2 (486 BAU/ml [175, 1133]), or ChAdOx1 nCoV-19 (190 BAU/ml [92, 504]). Patients receiving DMARD therapy showed reduced antibody levels as compared to individuals without: csDMARD only (480 BAU/ml [212, 1180]), bDMARD/tsDMARD mono- (167.50 BAU/ml [65.83, 462]), bDMARD/tsDMARD combination therapy (141.00 BAU/ml [56, 683]) versus non-immunosuppressed IMID patients (679 BAU/ml [246, 1347]) or HC (815.00 BAU/ml [479, 1532]). In the multivariable model, any immunosuppressive treatment was significantly associated with lower antibody levels. Compared to HC, only patients with connective tissue disease (CTD) had significantly impaired response to vaccination, when adjusted for age, vaccine strategy, time since vaccination and immunosuppression.
Conclusion: Immunosuppressive therapy as well as diagnosis of CTD significantly contributed to impaired humoral immune response after COVID-19 vaccination. Detection of anti-RD antibodies and early booster vaccination should be considered in these patients-at-risk.
To cite this abstract in AMA style:
Kastrati K, Mrak D, Weber P, Goethans L, Hana C, Simader E, Hummel T, Friedberg E, Aletaha D, Bonelli M, Radner H. Immunogenicity After Primary COVID-19 Vaccination in Patients with Immune-mediated Inflammatory Diseases: A Comparative Cohort Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/immunogenicity-after-primary-covid-19-vaccination-in-patients-with-immune-mediated-inflammatory-diseases-a-comparative-cohort-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunogenicity-after-primary-covid-19-vaccination-in-patients-with-immune-mediated-inflammatory-diseases-a-comparative-cohort-study/