Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: To assess the strength and duration of the immunological response to COVID-19 vaccines in patients treated with immunosuppressive medication for inflammatory arthritis.
Methods: Adult patients with a clinical diagnosis of rheumatoid arthritis (RA), spondyloartritis (SpA) or psoriatic arthritis (PsA) on treatment with any bDMARD, csDMARD, tsDMARD or prednisolone ( > 7,5 mg/day) intending to receive a COVID-19 vaccine and without contraindications for vaccination were eligible for inclusion in this observational study. A cohort of healthy controls consisting of health care workers has also been established. All subjects receive vaccines according to the national corona vaccination program, independent of the current study.
Serum samples have been obtained from participants before the first vaccine dose. Vaccinations and collection of blood samples 2-4 weeks after the second vaccine dose is still ongoing. Further serum samples will be collected for assessment every 3-6 months during the study period of 5 years. Samples are consecutively analysed for antibodies to SARS-CoV-2 using Microsphere Affinity Proteomics (MAP) (1). In order to further elucidate the immune response to COVID-19 vaccines, the T cell response will also be evaluated in a subgroup of patients and controls. Data regarding demographics, immunosuppressive medication and adverse events related to vaccination are recorded. Information regarding vaccination status and potential COVID-19 disease is obtained from relevant national health registers.
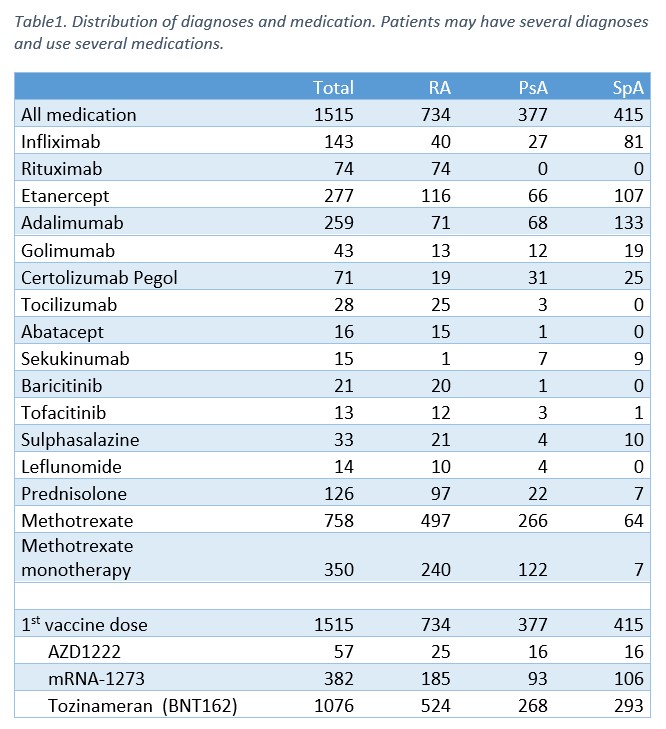
Results: From February 15 until May 27 2021, 1713 patients have been enrolled in the study. At present, 1515 patients have received at least one vaccine dose and 130 participants have provided samples for T cell analyses. The distribution of diagnoses and medication in the 1515 patients with at least one vaccine dose is shown in Table 1. 651 patients have received a second vaccine dose and 80 patients have serum samples available for analysis 2-4 weeks after the second vaccine dose. Further analyses of serological and cellular responses are currently ongoing, with first results expected summer of 2021.
Conclusion: Long-term immunosuppressive medication may differentially impact the COVID 19 vaccine response in patients being treated for arthritic diseases. Determining how strong and how long lasting the immune response is in different treatment groups will be crucial in decision-making with regard to adjustments in medication and to assess a possible need for re-vaccination. Further results on serological and cellular responses are expected late summer 2021 and will be of urgent importance to patients, health care systems and decision makers.
References
1. Holter JC et al. Proc Natl Acad Sci 2020
To cite this abstract in AMA style:
Jyssum I, Tveter A, Lund-Johansen F, Munthe L, Provan S, Jørgensen K, Grødeland G, Kro G, Warren D, Sexton J, Kvien T, Mjaaland S, Haavardsholm E, Vaage J, Syversen S, Goll G. Immune Responses to COVID-19 Vaccines in Patients Using Immunosuppressive Medication for Inflammatory Arthritis – An Observational Study of 1500 Patients [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/immune-responses-to-covid-19-vaccines-in-patients-using-immunosuppressive-medication-for-inflammatory-arthritis-an-observational-study-of-1500-patients/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immune-responses-to-covid-19-vaccines-in-patients-using-immunosuppressive-medication-for-inflammatory-arthritis-an-observational-study-of-1500-patients/