Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Deep B cell depletion has been confirmed to be the main mechanism for complete clinical response of SLE patients. We analyzed the peripheral blood of SLE patients in an in-vitro assay and found that CD47 was expressed in all B cell subtypes especially in plasmablasts which are CD20 negative. IMC-002 (IMM0306) is a mAb-Trap targeting CD20 and CD47, which has been demonstrated to have robust B cells depletion activity in phase I clinical trial for patients with non-hodgkin’s lymphoma. This study aims to investigate the treatment of IMC-002 on safety, SLE disease activity measures, proteinuria responses and multiple biomarkers in SLE patients who had not previously responded to standard of care.
Methods: As of 14th Apr. 2025 (cut-off date), 11 patients who completed 4-weeks administration (once weekly) of IMC-002 and at least one SLEDAI-2K evaluation in Phase 1b were included in this analysis, 7 patients in 0.8mg/kg dose cohort were followed up for 16-24 weeks, 4 patients in 1.2mg/kg dose cohort were followed up for 4-8 weeks. Moderate to severe active SLE was defined as Class A manifestations in one or more organ systems and/or Class B manifestations in two or more organ systems and SLEDAI≥6, ANA or Anti-dsDNA positive. The efficacy endpoint was SLE Responder Index-4 (SRI-4) evaluation for every 12 weeks in Phase 1b. Other efficacy assessments included BILAG-2004, SLEDAI-2K, PGA and laboratory tests (24-hour urine protein, anti-dsDNA antibody, C3/C4 and IgG/IgA/IgM, B cells count, etc.)
Results: By the data cut-off date, in the 0.8 mg/kg dose cohort, 2 out of 7 patients reached SRI-4 at Week 12, and all patients did not reach 24-week SRI-4 evaluation time; in the 1.2 mg/kg dose cohort, all patients did not reach 12-week SRI-4 evaluation time. 4 patients (57.1%) in the 0.8 mg/kg dose cohort and 4 patients (100%) in 1.2 mg/kg dose cohort showed SLEDAI-2K improvement by≥4 with PGA decrease; 1 patient SLEDAI-2K scored from 8 (baseline) to 0 (W8) in the 0.8 mg/kg dose cohort and 1 patient from 8 (baseline) to 0 (W4) in the 1.2 mg/kg dose cohort (Figure 1). All patients had improvements or no changes in BILAG-2004, and no PGA score worsening in all patients except 1 patient had 0.4 increasement. The 24-hour urine protein were significantly decreased by 32% ~ 62% from baseline during the administration period in all the 5 patients with proteinuria≥0.5g/24h at baseline. Anti-dsDNA antibody in all patients (6/6) showed a significant decrease trend. C3/C4 recovered to normal in 75% patients (3/4) with low complement at baseline. Complete deletion (defined as less than 5 cells/μL) of CD19+ B cells and plasma blasts in all 11 patients was achieved from end of injection (EOI) to latest visit time (up to W20) (Figure 2).
Conclusion: Results of this study suggest that treatment with IMC-002 is effective across multiple organ systems in patients with active SLE. IMC-002 showed deep and persistent B cell depletion in active SLE patients.
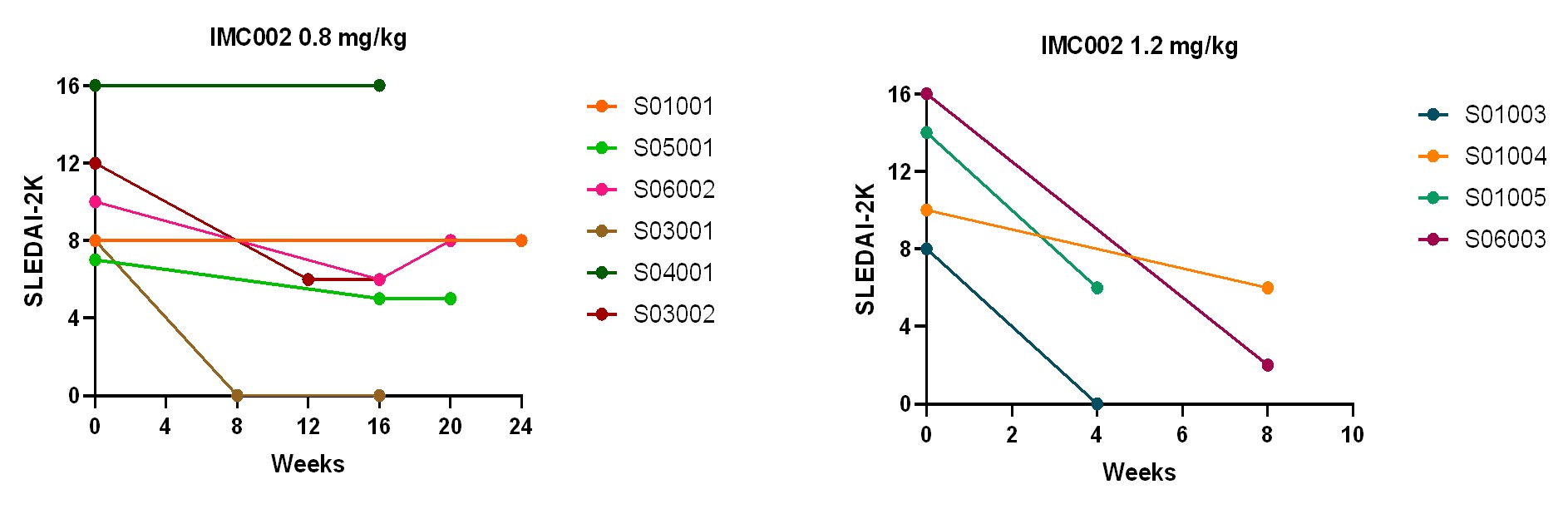 Figure 1. SLEDAI-2K evaluation from first dose to the latest visit at the time of data cutoff
Figure 1. SLEDAI-2K evaluation from first dose to the latest visit at the time of data cutoff
.jpg) Figure 2. B lymphocyte depletion from first dose to latest available data at the time of data cutoff
Figure 2. B lymphocyte depletion from first dose to latest available data at the time of data cutoff
To cite this abstract in AMA style:
Yao H, Tian W, Zheng Q, Chen M, Jiang G, Liu Z, Nie Y, Wu R, Zheng Z, Li Z. IMC-002 (IMM0306), a First-in-Class Bi-specific Fusion Protein, Demonstrates Improvements in Systemic Lupus Erythematosus (SLE) Disease Activity Measures and Biomarkers in Patients with Moderate to Severe Active SLE in the Open-label Phase 1b/2 Study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/imc-002-imm0306-a-first-in-class-bi-specific-fusion-protein-demonstrates-improvements-in-systemic-lupus-erythematosus-sle-disease-activity-measures-and-biomarkers-in-patients-with-moderate-to-se/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/imc-002-imm0306-a-first-in-class-bi-specific-fusion-protein-demonstrates-improvements-in-systemic-lupus-erythematosus-sle-disease-activity-measures-and-biomarkers-in-patients-with-moderate-to-se/
