Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite significant advances in the management of patients with lupus nephritis (LN), a significant proportion of patients either do not respond to first-line immunosuppressive drugs, or relapse after initial remission. Iguratimod is a novel disease modifying anti-rheumatic drug that has been approved for treating rheumatoid arthritis in East Asia and has shown benefits in lupus animal model in our previous research. The aim of this study was to make a preliminary observation on the efficacy and safety of iguratimod in treating refractory LN patients.
Methods: We have enrolled adult refractory LN patients since 2015, who were eligible if they experienced at least two times of failure or relapse before enrollment. Failure was defined as no response to one certain immunosuppressive drug for at least six months. After enrollment, we simply switched their previous immunosuppressant to iguratimod (25 mg twice per day) and keeping all other medications. Complete/partial remission (PR/CR) at Week 24 was used as the primary outcome. An extended follow-up would continue once the patient achieved remission.
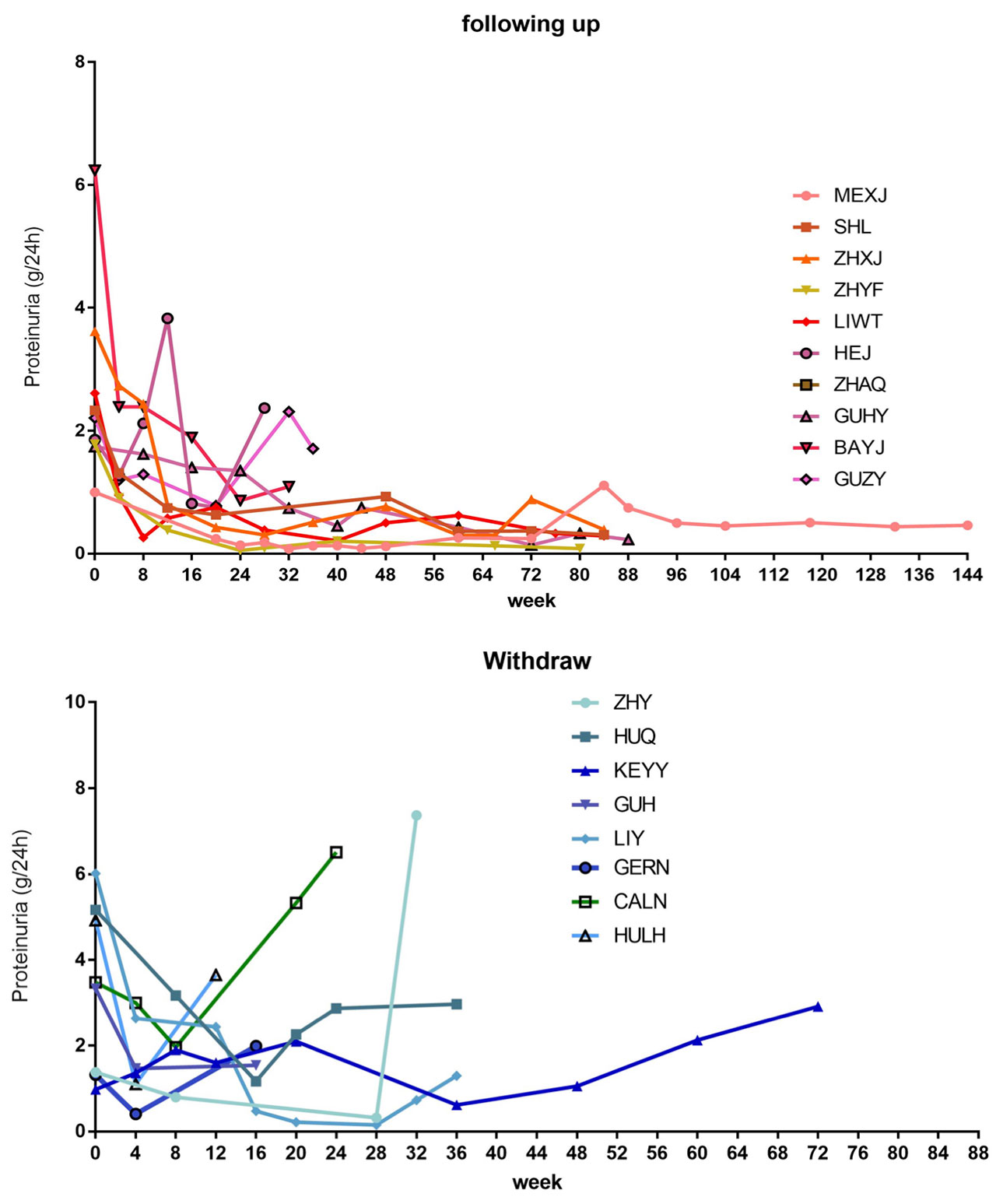
Results: A total of 20 refractory LN patients had been enrolled (18 female and 2 male patients) since 2015. At enrollment, the median proteinuria was 2.59g/24h (interquartile range, IQR: 1.52-4.92g/24h). None of them had observable extra-renal symptoms. All of them had biopsy-proven LN (class Ⅲ/Ⅳ/Ⅴ) and two patients agreed repeated biopsy before switching to iguratimod. The median prednisone dosage was 10mg/d (IQR: 0-10mg/d). One withdrew the consents. Of the remaining 19 patients, the renal response rate was 84.2% at week 24. The median response duration (MRD) was 12 weeks and the IQR was 10-18 weeks. In follow-up of the 16 remitted patients, 25% (4/16) patients had renal relapse (median relapse duration: 32 weeks) and 4 patients exited the study for other reasons (one had extra-renal flare, one had severe adverse event, one lost of follow-up another was for incompliance). For the remaining 9 patients who were still in follow-up, the median follow-up was 84 weeks (IQR: 42-118 weeks), with 4 having CR and 5 PR.
Conclusion: Out study provided preliminary but promising clinical evidence for iguratimod in treating refractory LN patients. A large randomized clinical trial is needed to establish its safety and efficacy for refractory LN.
To cite this abstract in AMA style:
Yan Q, Bao C, Kang Y, Fu Q, Wang R. Iguratimod Is an Alternative Option for Refractory Lupus Nephritis: A Preliminary Observational Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/iguratimod-is-an-alternative-option-for-refractory-lupus-nephritis-a-preliminary-observational-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/iguratimod-is-an-alternative-option-for-refractory-lupus-nephritis-a-preliminary-observational-study/