Session Information
Date: Friday, November 6, 2020
Title: Patient Outcomes, Preferences, & Attitudes Poster I: RA, Spondyloarthritis, & OA
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Systemic Juvenile Idiopathic Arthritis (SJIA) can cause severe and chronic multisystem involvement. Medical therapies including high-dose corticosteroids can have significant side effects affecting sleep. The primary aims are to validate Patient-Reported Outcomes Measurement Information System (PROMIS) surveys- sleep disturbance (survey A) and sleep impairment (survey B). Our hypothesis is that current steroid drug treatment cause worse sleep.
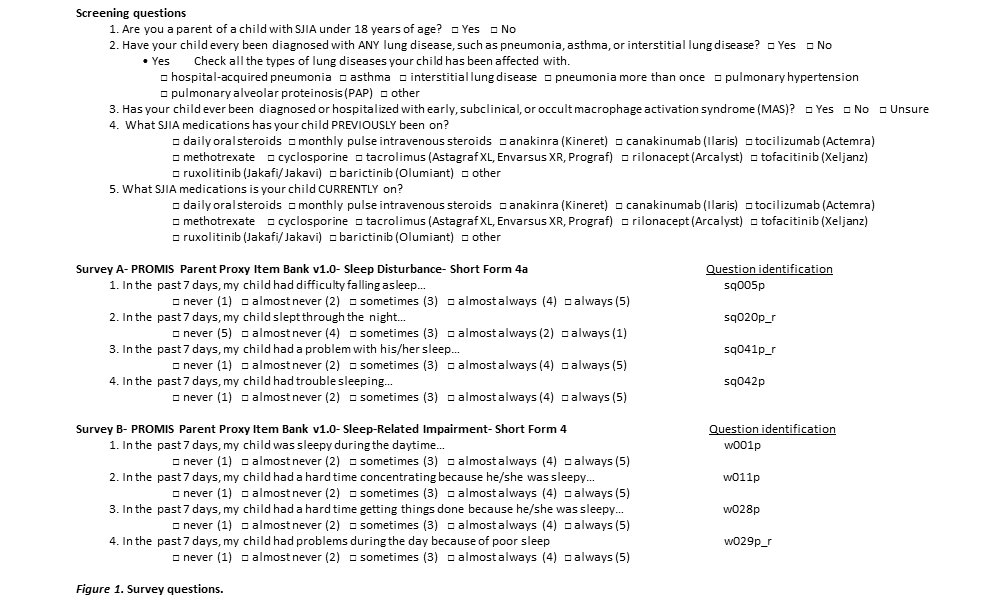
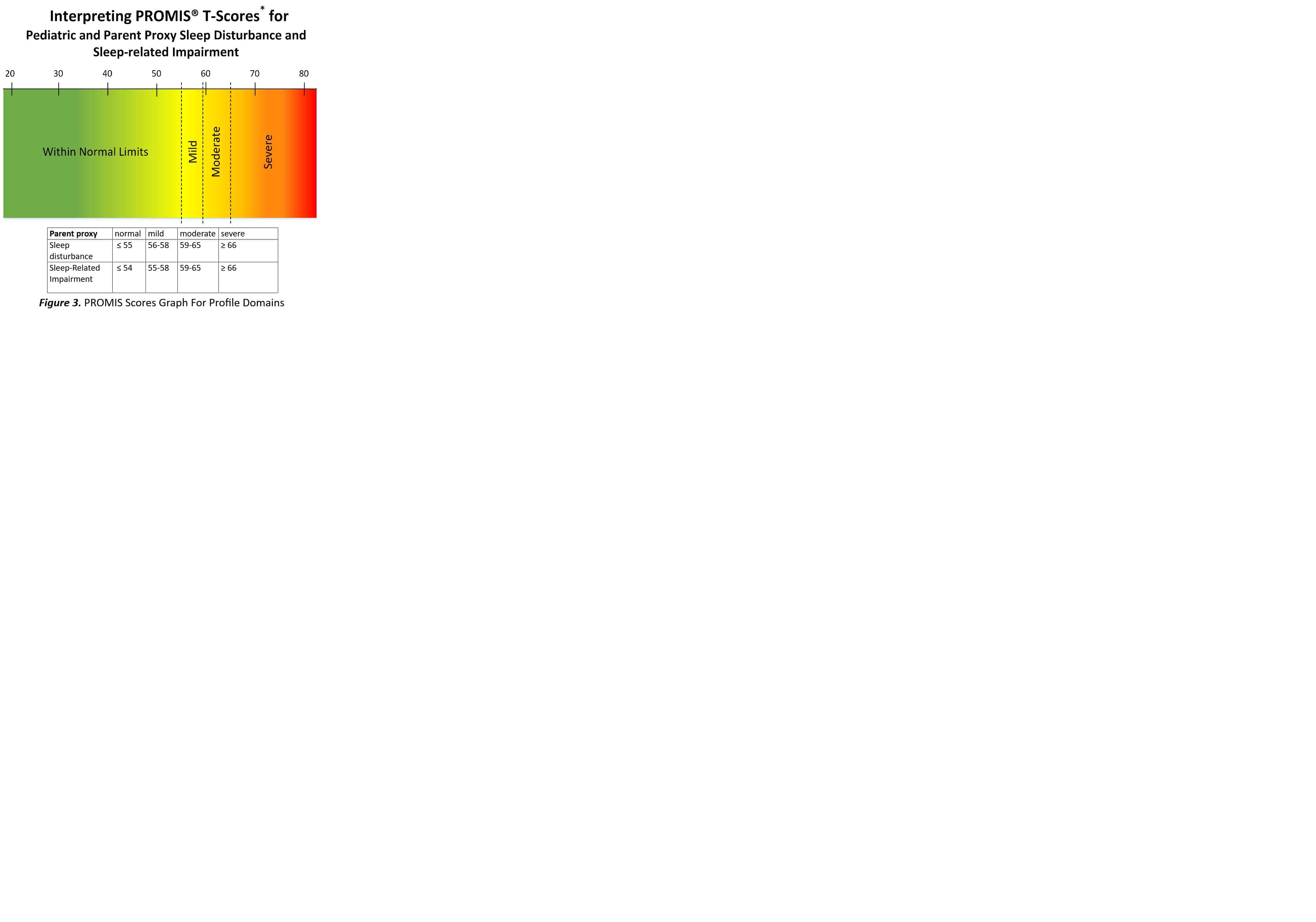
Methods: Patients were identified through the CCHMC JIA database and Systemic JIA Foundation, and included parents or guardians of SJIA patients under 18 years of age at the time of the survey. Survey invitations were mailed or e-mailed, and de-identified data were stored in Research Electronic Data Capture. The two surveys are listed in figure 1 and include demographic screening questions and past and current drug treatments. In performing descriptive statistical analyses, PROMIS surveys are represented by t-scores, defined as standardized scores with a mean of 50 and a standard deviation (SD) of 10. Between-group comparisons were done with t-tests. Severity of sleep problems were determined by the corresponding PROMIS Scores Graph For Profile Domains (figure 3).
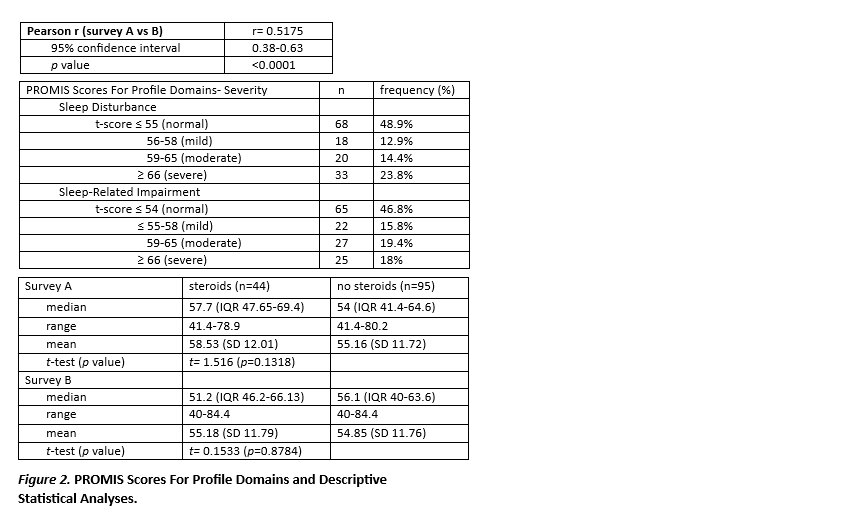
Results: 139 participants were included in the survey. There was reasonable correlation between survey A and B (r=0.5175). For survey A, 51.1% (n=71) of patients reported some level of sleep disturbance; 12.9% were mild, 14.4% were moderate, and 23.8% were severe. For survey B, 53.2% (n=74) of patients reported sleep impairment; 15.8% were mild, 19.4% were moderate, and 18% were severe. For survey A, the steroid treatment group showed a mean of 58.43 (SD 12.01), a median of 57.7 (IQR 47.65-69.4), and a range of 41.4 to 78.9. The no steroid treatment group had a mean of 55.16 (SD 11.72), a median of 54 (IQR 41.4-64.6), and a range of 41.4 to 78.9. T-test treatment group comparison was not significant (p=0.13). In survey B, patients treated with steroids showed a mean of 55.18 (SD 11.79), a median of 51.2 (IQR 46.2-66.13), and a range of 40 to 84.4. The no steroid treatment group had a mean of 54.85 (SD 11.76), a median of 56.1 (IQR 40-63.6), and a range of 40 to 84.4 There was not statistical significance between treatment groups (p=0.88) (figure 2).
Conclusion: In conclusion, a majority of patient with SJIA have clinically significant sleep disturbance and/or impairment. However, current steroid treatment did not have statistical significance for causing worse sleep. Sleep problems interfere with quality of life and future endeavors include identification of factors that correlate with higher scores.
To cite this abstract in AMA style:
Nguyen K, Towe C, Yasin S, Grom A, Brunner H, Schulert G. Identifying Sleep Problems in Systemic Juvenile Idiopathic Arthritis (SJIA) Patients with Patient-reported Outcomes (PRO) Questionnaires [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/identifying-sleep-problems-in-systemic-juvenile-idiopathic-arthritis-sjia-patients-with-patient-reported-outcomes-pro-questionnaires/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identifying-sleep-problems-in-systemic-juvenile-idiopathic-arthritis-sjia-patients-with-patient-reported-outcomes-pro-questionnaires/