Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The purpose of this analysis was to assess the frequency of inadequate response (IR) over 1 year from biologic initiation among ankylosing spondylitis (AS) patients in the United States using a claims-based algorithm that was originally developed and validated for rheumatoid arthritis1. Baseline factors associated with IR to biologic were also analyzed.
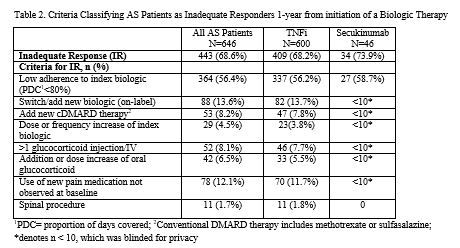
Methods: This was a retrospective cohort study using claims data from the HealthCore Integrated Research Database (HIRD®). Adult patients with AS who initiated a biologic (TNFi: adalimumab, certolizumab, etanercept, golimumab, infliximab; IL-17: secukinumab) from 7/1/2016 to 8/31/2018 and had continuous enrollment ≥6 months before and ≥12 months after index date (date of first biologic claim) were included. The index biologic was defined as the first biologic prescribed during the study time period. Patients were identified as having IR to their index biologic if during the 12 months after index date they had one or more of the following: low adherence (defined as proportion of days covered (PDC)< 80%), switched/added new biologic, added a new conventional disease modifying anti-rheumatic drugs (cDMARDs) (methotrexate and/or sulfasalazine), increased dose/frequency of biologic, >1 glucocorticoid injection/infusion, addition or dose increase of oral glucocorticoids, used a new pain medication, or had a spinal procedure. Baseline patient characteristics were compared between responders and IRs using Chi-square tests for categorical variables and t-tests for continuous variables. A multivariable logistic regression model was constructed to identify baseline characteristics associated with IR to index biologic.
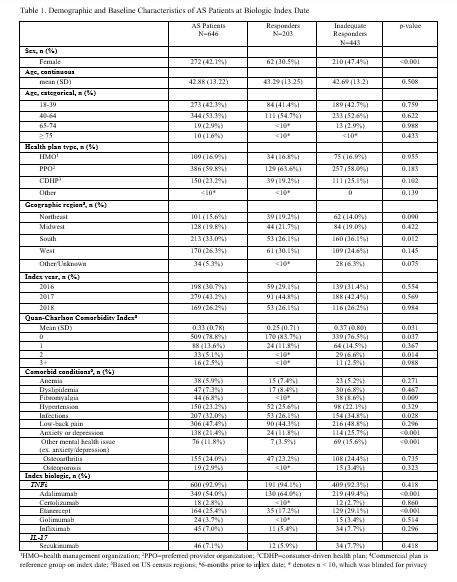
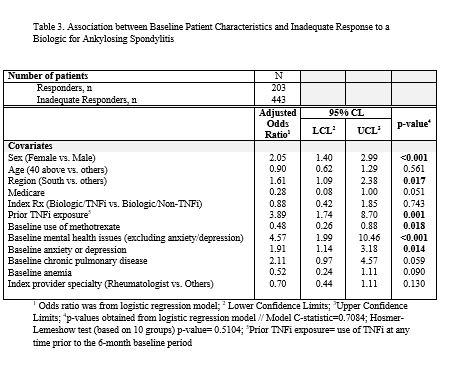
Results: A total of 646 AS patients were included in this analysis. Mean age was 43 years, 58% were male, 93% initiated a TNFi, and 7% initiated an IL-17 as their index biologic (Table 1). Over the 1-year follow-up period, 69% of AS patients had an IR to their biologic: 56% of patients had low adherence, 14% switched/added a new biologic, 8% added a new cDMARD, 4% had a dose/frequency increase of their index biologic, 8% had >1 glucocorticoid injection/infusion, 6% had an addition/dose increase of oral glucocorticoids, 12% used a new pain medication, and 2% had a spinal procedure (Table 2). Inadequate responders were more likely to be female (odds ratio (OR)=2.05; p< 0.001), have anxiety or depression (OR=1.91; p=0.014) and other mental health issues (OR=4.57; p< 0.001), and live in the South (OR=1.61; p=0.017); while patients with baseline use of methotrexate were more likely to be responders (OR=0.48; p=0.018) (Table 3). Prior exposure to TNFi was associated with a 3.89-fold greater odds of non-response (p=0.001).
Conclusion: Over 68% of AS patients had an inadequate response to their index biologic 1 year after initiation, mostly driven by low adherence. Health plan claims data appears useful to classify inadequate responders in AS and additional research should be done to further validate this claims-based algorithm in a clinical setting.
To cite this abstract in AMA style:
Hunter T, Grabner M, Isenberg K, Shan M, Teng C, Lisse J, Curtis J. Identifying Inadequate Response Among Ankylosing Spondylitis Patients Prescribed Biologics in a Real-world Commercially Insured Adult Population in the United States [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/identifying-inadequate-response-among-ankylosing-spondylitis-patients-prescribed-biologics-in-a-real-world-commercially-insured-adult-population-in-the-united-states/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identifying-inadequate-response-among-ankylosing-spondylitis-patients-prescribed-biologics-in-a-real-world-commercially-insured-adult-population-in-the-united-states/