Session Information
Date: Tuesday, November 14, 2023
Title: (2257–2325) SLE – Diagnosis, Manifestations, & Outcomes Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE) is a novel clinical outcome assessment (COA) for SLE randomized controlled trials (RCTs), being developed by an international clinician-industry-patient taskforce. Current COA development guidelines require that outcome measures used in RCTs should capture treatment effects relevant to how patients “feel, function or survive” to support regulatory approval (1). The aim of this study was to identify domains to be considered for inclusion in TRM-SLE from the perspective of expert SLE clinicians and patients based on this paradigm.
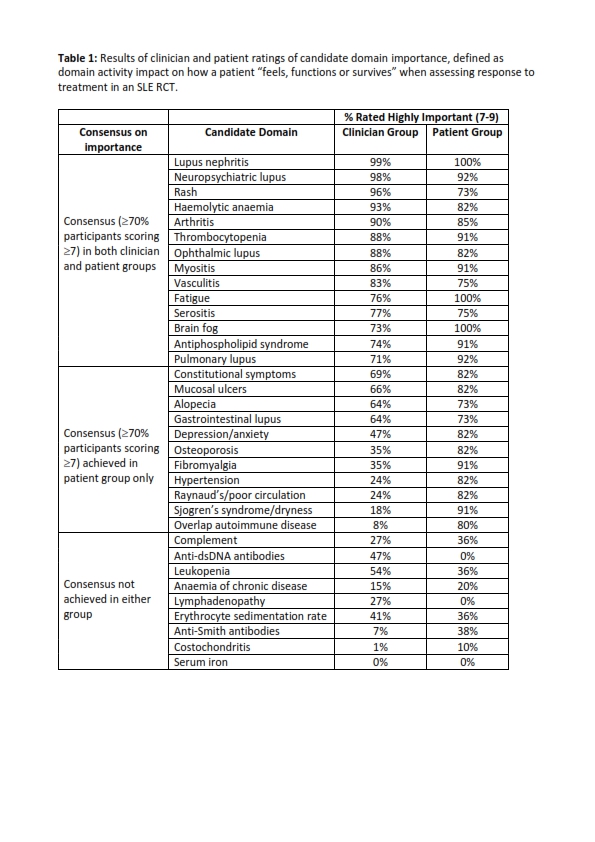
Methods: Candidate domains were identified via surveying a multinational panel of clinicians, patients and industry representatives. Domains were then rated in a modified Delphi study (two online survey rounds separated by a discussion meeting) in which clinicians with SLE expertise and patient advisory panellists were invited to rate the “importance” of candidate domains on a 1-9 scale (where 1 = not important and 9 = critically important). Importance was defined as domain activity having an impact on how a patient “feels, functions or survives”, when assessing response to treatment in an SLE RCT. A literature review summarising associations of each candidate domain with key SLE symptoms, functional impact, damage and mortality was provided to participants to support participant ratings. The consensus threshold was prespecified as a rating of at least 7 by 70% of participants in both clinician and patient groups.
Results: The domain generation survey yielded 64 nominations, grouped into a core list of 34 candidate domains. Of 118 invitees to the modified Delphi study, 87 clinicians with a median (interquartile range; IQR) of 21 (11, 30) years’ experience and 13 patients/representatives with a median (IQR) disease duration of 19 (16,33) years participated, with representation from six continents. Of the 34 candidate domains, 6 met the consensus threshold after Round 1 of voting, increasing to 14 after Round 2 (Table 1). An additional 11 domains met consensus in the patient group only. Notably, some domains meeting consensus on importance (e.g. fatigue) are not measured in current primary SLE trial endpoints, while others (e.g. anti-dsDNA, complement) included in current response measures did not reach consensus for inclusion.
Conclusion: When rated for importance, consensus was reached in both SLE clinician and patient groups on multiple candidate domains for inclusion in a novel COA for SLE trials. Discrepancies between clinician and patient perspectives were also identified. Important domains will next be rated on other characteristics relevant to inclusion in a COA (appropriateness, representation and measurability), with selected domains to proceed to definition of response measurement.
References:
1. FDA Patient Focused Drug Development Guidance: Selecting, Developing or Modifying Fit-for-Purpose Clinical Outcome Assessments. https://www.fda.gov/media/159500/download
To cite this abstract in AMA style:
Connelly K, Koelmeyer R, Ayton D, Barrallon R, Eades L, Golder V, gregory K, May J, Kandane-Rathnayake R, Mydin M, Akther M, Anzum A, Andersen J, Brunner H, Aranow C, Arnaud L, Askanase A, Barbey C, Buie J, Burke L, Cornet A, Costenbader K, Dall'Era M, Merrill J, Dantata K, Friedman A, Furie R, Garces S, Hojnik M, Kalunian K, Maller J, Marquis P, Mosca M, Noss E, Pons-Estel G, Simon L, Smith E, Sorrentino A, Stevens A, Stojan G, Sun Y, Tanaka Y, Vital E, van Vollenhoven R, Werth V, Morand E. Identifying Important Domains for Inclusion in a Novel Treatment Response Measure for Systemic Lupus Erythematosus (TRM-SLE): Results of a Modified Delphi Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/identifying-important-domains-for-inclusion-in-a-novel-treatment-response-measure-for-systemic-lupus-erythematosus-trm-sle-results-of-a-modified-delphi-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identifying-important-domains-for-inclusion-in-a-novel-treatment-response-measure-for-systemic-lupus-erythematosus-trm-sle-results-of-a-modified-delphi-study/