Session Information
Date: Saturday, November 12, 2022
Title: Health Services Research Poster I: Lupus, RA, Spondyloarthritis and More
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Data is lacking regarding modern real-world practices and disease outcomes of individuals with childhood-onset systemic lupus erythematosus (cSLE) and lupus nephritis, especially across multiple centers. However, researchers also lack approaches to identify end-stage kidney disease (ESKD) in patients with cSLE utilizing claims-based algorithms. Our objective was to determine optimal definitions that can be used to identify patients with cSLE, lupus nephritis, and ESKD using a large, real-world administrative claims database.
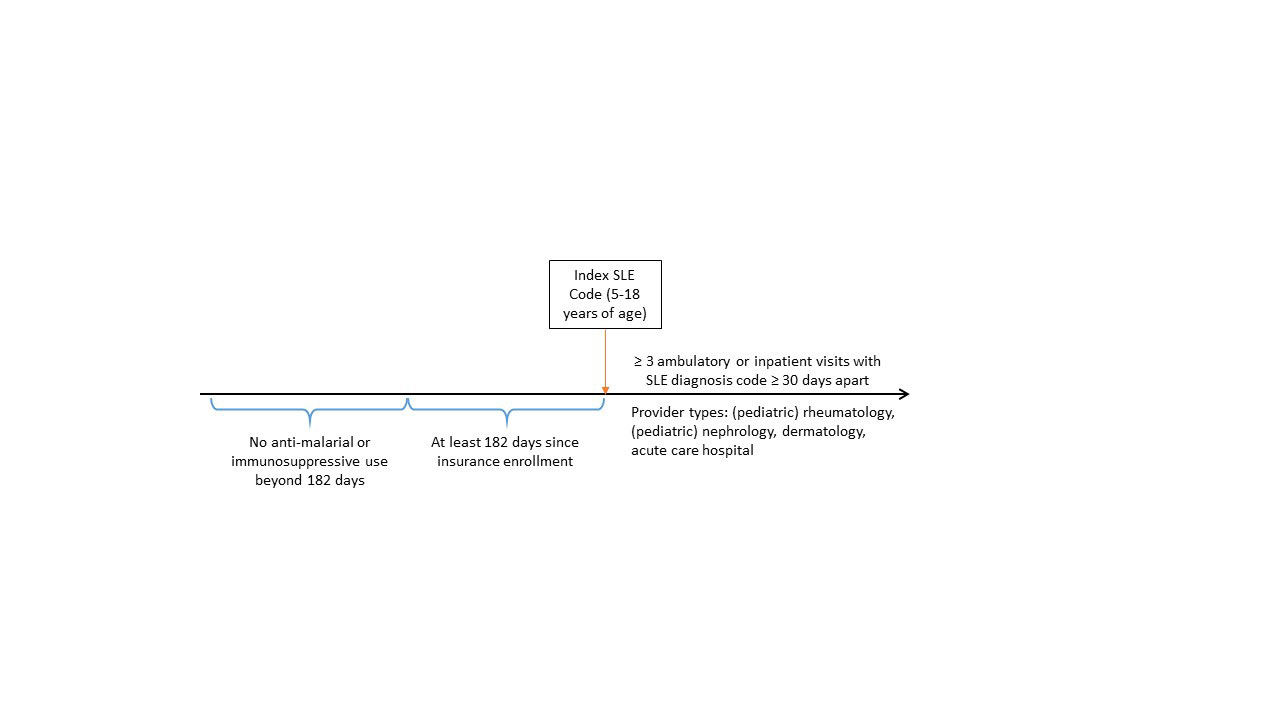
Methods: We utilized administrative claims data from IBM MarketScan Commercial and 8-State Medicaid Databases from 2006-2020. We adapted previously validated definitions to identify patients with cSLE: 1) ≥ 3 ambulatory or inpatient claims with ICD-9 code 710.0 or ICD-10-CM code M32.* (excluding M32.0 for drug-induced lupus) with at least 30 days between each code; 2) codes restricted to provider type pediatric rheumatology, rheumatology, pediatric nephrology, nephrology, dermatology, or acute care hospital; and 3) age ≥ 5 and < 18 years at the time of the first SLE code. To define incident diagnoses, we selected patients with: 1) at least 182 days between insurance enrollment and the first SLE code; and 2) no evidence of prior anti-malarial or immunosuppressive use more than 182 days before the first SLE code (Figure 1). We then adapted previously validated definitions to select the subgroup of cSLE patients with evidence of lupus nephritis using ≥ 2 ambulatory or inpatient claims with ICD-9 codes 580.*-586.*, 791.0 or ICD-10-CM codes M32.14, M32.15 with at least 30 days between each code. Finally, we defined presence of ESKD using: 1) procedure and diagnosis codes for dialysis from any encounter; 2) procedure and diagnosis codes for kidney transplant from any encounter; or 3) ≥ 3 claims with diagnosis codes for ESKD from any encounter type. We considered each ESKD criteria separately and as a composite if any of the 3 criteria were met. We calculated the frequency of patients meeting each of these criteria.
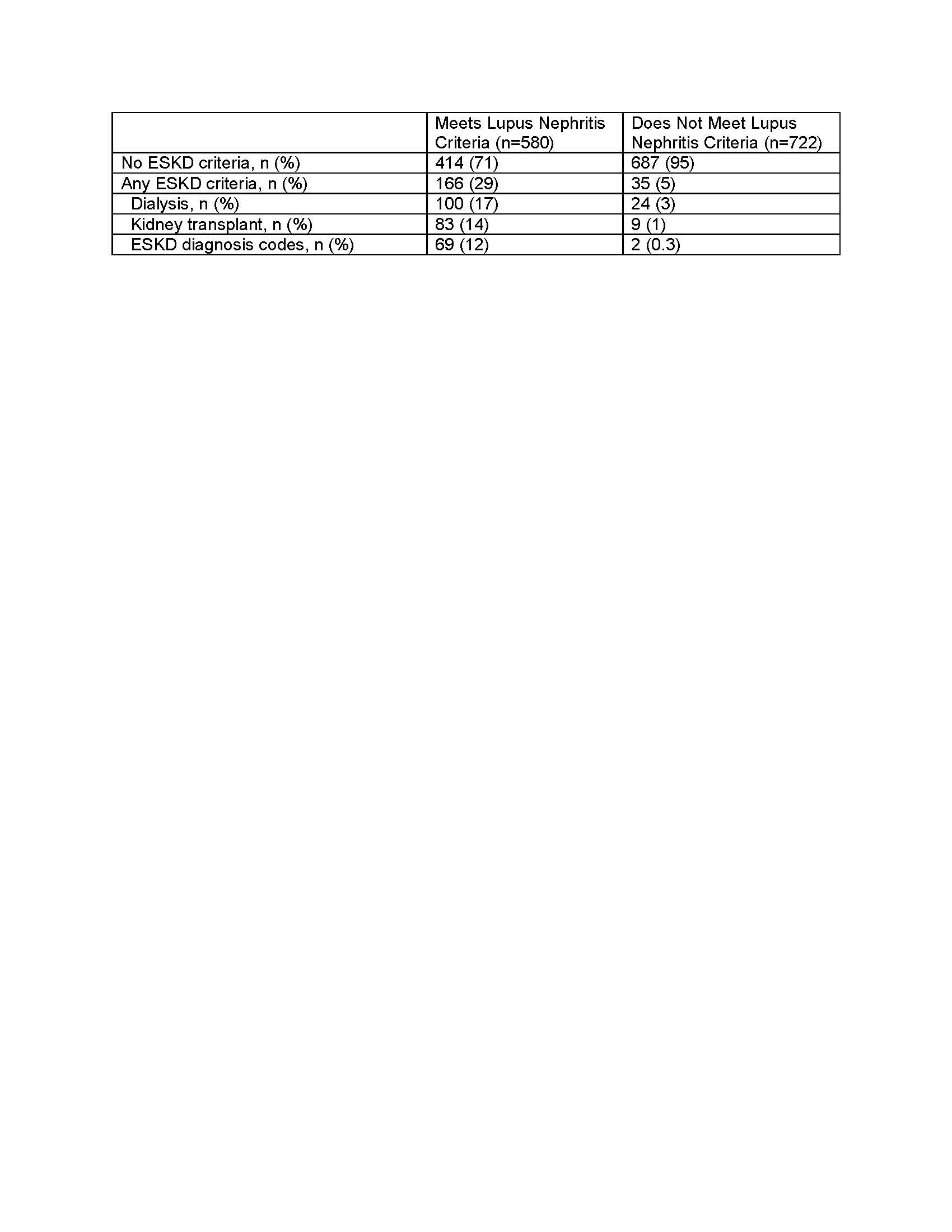
Results: From 2006-2020, we identified 2,590 individuals receiving care for cSLE in the IBM MarketScan Commercial and 8-State Medicaid Databases, of which 1,302 met criteria for an incident diagnosis. Definition for lupus nephritis was met in 580 (45%) of incident cSLE cases. Of individuals meeting the lupus nephritis definition, 166 (29%) had evidence of ESKD: 100 (17%) with dialysis, 83 (14%) with kidney transplant, and 69 (12%) with other ESKD diagnosis codes. Only 35 (4%) of 722 individuals with incident cSLE who did not meet lupus nephritis definition had evidence of ESKD: 24 (3%) with dialysis, 9 (1%) with kidney transplant, and 2 (0.3%) with other ESKD diagnosis codes (Table 1).
Conclusion: This study of individuals with evidence of cSLE and lupus nephritis within a US administrative claims database revealed a relatively high frequency of meeting ESKD criteria among cSLE patients. We identified few individuals with evidence of ESKD who did not meet previously validated lupus nephritis definitions; nevertheless, ESKD care should be included in the definition of lupus nephritis when using administrative claims algorithms.
To cite this abstract in AMA style:
Smitherman E, Chahine R, Hersh A, Curtis J. Identifying Childhood-Onset Systemic Lupus Erythematosus, Lupus Nephritis, and End-Stage Kidney Disease Definitions Within an Administrative Claims Database [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/identifying-childhood-onset-systemic-lupus-erythematosus-lupus-nephritis-and-end-stage-kidney-disease-definitions-within-an-administrative-claims-database/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identifying-childhood-onset-systemic-lupus-erythematosus-lupus-nephritis-and-end-stage-kidney-disease-definitions-within-an-administrative-claims-database/