Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: SLE flare is a clinically and regulatory relevant outcome, yet limited markers currently exist that predict its risk. We used baseline whole blood/serum samples and phenotypic data from four phase 3 (SLE) trials (NCT01205438, NCT01196091, NCT03616964, NCT03616912) to conduct comprehensive analyses for severe flare predictors.
Methods: The analyses examined 55,757 molecular markers (whole blood RNA=16,641, serum protein=2,941 and DNA methylation=36,175) from placebo (i.e. background standard of care medication) treated patients (n=1266 pts). Risk of having a severe flare as measured by the SLEDAI Flare Index through week 52 was considered as the clinical outcome of interest.
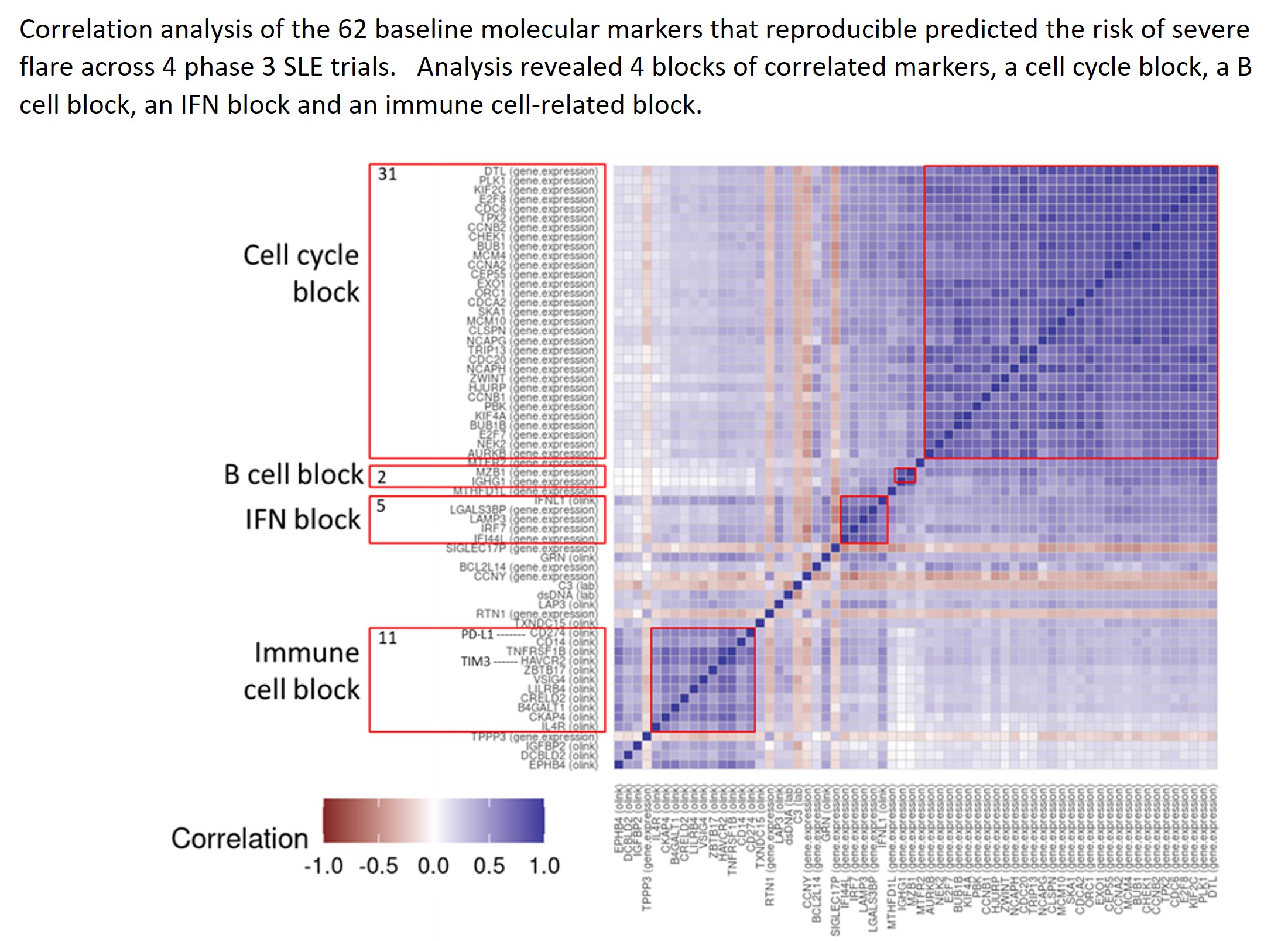
Results: : Two markers previously identified as predictive of severe SLEDAI flare were confirmed in all 4 trials (presence of anti-dsDNA antibodies and IFI44L RNA levels; Hoffman et al, 2017). In addition, 62 novel molecular markers were consistently associated with elevated risk of SLEDAI severe flares with a posterior probability greater than 0.5 and a consistent direction of effect, comprising 44 RNA-based and 18 protein-based markers. Correlation analysis of the 64 markers identified 4 functional consensus clusters of correlated upregulated genes, including a cell cycle block (31 markers), B cell block (2 markers), interferon-related block (5 markers) and an immune-related block (11 markers) (Figure). We then used single cell CITE-seq analysis on a separate cohort of SLE patients (n=15) to identify specific blood cells that had the highest levels of the 62 flare markers, revealing plasma cells, followed by monocytes and plasmacytoid dendritic cells. Functional enrichment analysis using the LINCS database, a library of high-throughput drug and genetic perturbations screens across cell lines in vitro revealed a significant enrichment (p < 10-10) of CDK4/6 inhibitors associated with the set of 62 SLE flare markers. Both CDK4 shRNA and the CDK4/6 inhibitor palbociclib were identified as perturbations that reverse expression of the 62 molecular flare markers in-vitro, while IFNα and IFNγ were identified as perturbations that enhance the expression of the 62 flare markers.
Conclusion: This work enhances our understanding of molecular predictors for severe SLE flares in the context of clinical trials. The findings also suggest potential new pathways for targeted treatment strategies.
To cite this abstract in AMA style:
Linnik M, Rocha G, Gemperline D, Dow E, Preuss C, Masson H, Ellis O, Accioly A, Hojnik M, Rubtsova K, Benschop R, Chambers M, Genovese M, Higgs R. Identification of Co-expressed Molecular Markers That Predict Risk of Severe Flare in Patients with Systemic Lupus Erythematosus (SLE) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/identification-of-co-expressed-molecular-markers-that-predict-risk-of-severe-flare-in-patients-with-systemic-lupus-erythematosus-sle/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-co-expressed-molecular-markers-that-predict-risk-of-severe-flare-in-patients-with-systemic-lupus-erythematosus-sle/