Session Information
Date: Monday, November 11, 2019
Title: 4M117: RA – Treatments III: Cardiovascular Disease & Readmissions (1890–1895)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Hydroxychloroquine (HCQ) has known immunomodulatory effects and mechanistic actions that may provide cardioprotective effects. To date, cardiovascular (CV) outcomes in elderly patients receiving HCQ in clinical practice have not been evaluated. This is important since the majority of patients with CV disease in the U.S. are adults ≥ 65 years. We hypothesized that the use of HCQ is protective for major adverse cardiovascular events (MACE) defined as acute admissions for stroke, myocardial infarction or heart failure.
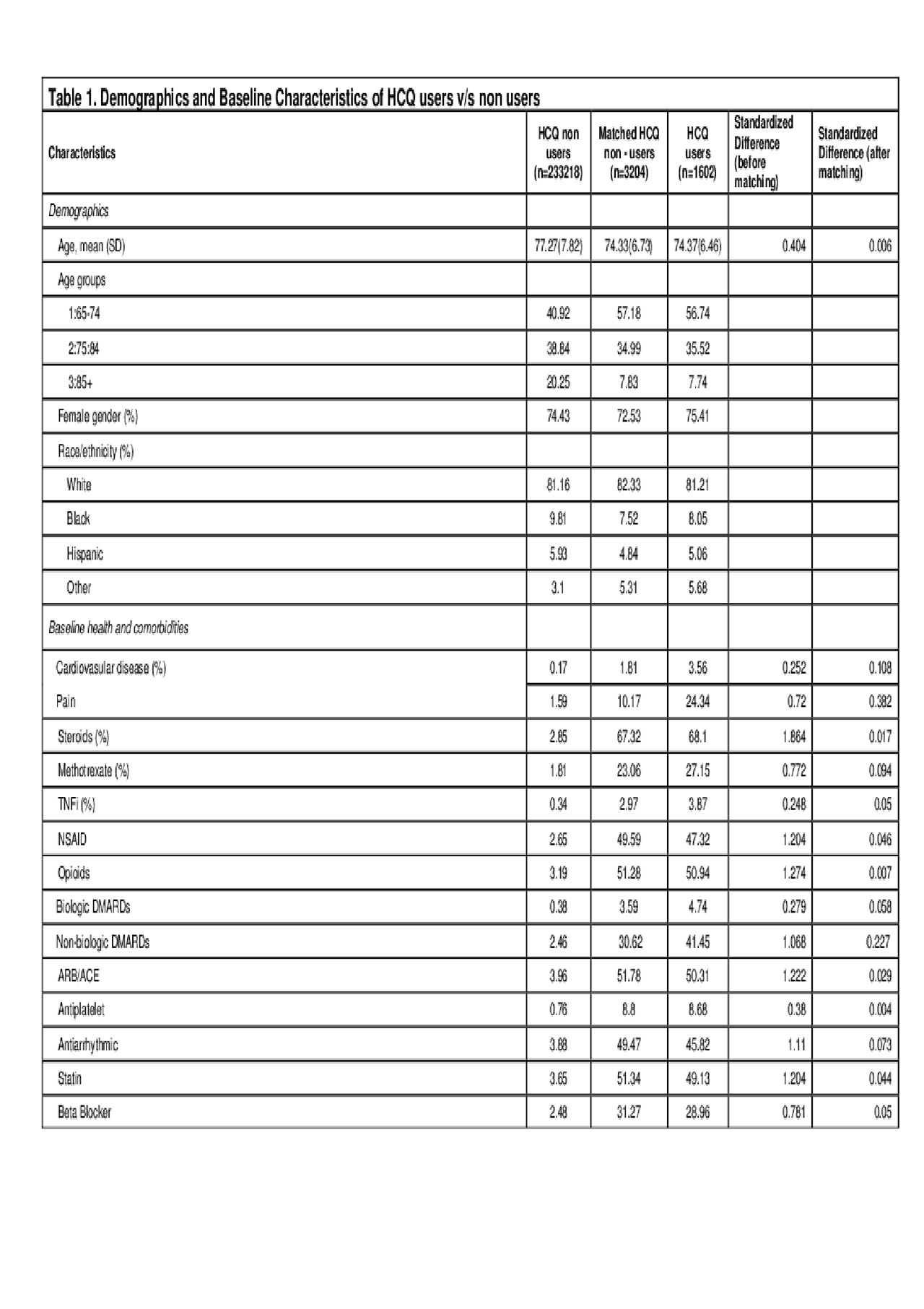
Methods: We compared the risk of MACE in a retrospective cohort of Medicare beneficiaries aged ≥65 years with rheumatoid arthritis (RA) who initiated HCQ (exposed) and who did not initiate HCQ (non-exposed) between 2014 and 2016. RA was defined by the following ICD codes – 714.0, 714.2, M05.xx, M06.4 and M06.1. Data sources included Medicare Parts A, B, and D claims for a 5% random sample of beneficiaries. The index date for the exposed was defined by the date of the first HCQ fill. Each exposed user was matched to 2 non-users of HCQ using propensity score derived from patient baseline characteristics including demographics, comorbid conditions like prior CV disease, and other medication use (cardioprotective agents like ACE inhibitors, antiplatelets, statins and beta blockers, NSAIDs, opioids, steroids, methotrexate, and tumor necrosis factor alpha inhibitors [TNFi]) (Table 1). Patient follow-up started at the index date and continued until the first MACE, end of HCQ use or the end of the observation period (December 31, 2016). We compared incident MACE using Kaplan-Meier analysis on matched patients. The primary outcome was the occurrence of MACE, and the secondary outcome was the composite of MACE and all-cause mortality. Cox proportional hazards model was used to compare the hazards of MACE events between HCQ users to non-users.
Results: We found 1602 eligible RA patients with incident HCQ use and matched them to 3204 RA patients who did not use HCQ over the study period. The mean age of HCQ user cohort was 74.37 years and of the non-users was 74.73. 75.41% of the HCQ users were females compared to 72.53% in the non-users. The mean follow-up time was 1.7 years in non-users and 1 year in HCQ users. During the observation period, 329 MACE events occurred, 54 in HCQ exposed (3.51 per 100 patient-years) and 275 in the non-exposed group (incidence rate of 5.38 per 100 patient-years. Multivariate Cox regression model showed a hazard ratio (HR) of 0.47 (95% CI: 0.35-0.63, p< 0.0001) for MACE for HCQ users versus non-users. Compared to people aged 85+ years, those aged 75 to < 85 (HR 0.73, 95% CI: 0.51 – 1.04), or those aged 65 to < 75 (HR 0.59, 95% CI: 0.42 – 0.84) years had a lower hazard of MACE. Similarly, the HR for composite outcome of MACE and all-cause mortality was 0.40 (95% CI: 0.32-0.51, p< 0.0001) for users compared to non-users.
Conclusion: Our study suggests that incident HCQ use is associated with a 53% lower risk of MACE events compared to non-users in an elderly population of patients with RA. Based on our study, future larger trials can be designed looking at the efficacy of a well-tolerated and relatively inexpensive drug on CV outcomes in the general population.
To cite this abstract in AMA style:
Iyer P, Gao Y, Girotra S, Curtis J, Vaughan-Sarrazin M, Singh N. Hydroxychloroquine Use Is Associated with a Lower Risk of Major Adverse Cardiovascular Events in Medicare Recipients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/hydroxychloroquine-use-is-associated-with-a-lower-risk-of-major-adverse-cardiovascular-events-in-medicare-recipients-with-rheumatoid-arthritis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-use-is-associated-with-a-lower-risk-of-major-adverse-cardiovascular-events-in-medicare-recipients-with-rheumatoid-arthritis/