Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Preterm delivery (PTD) occurs 2-3 times more frequently in patients with systemic lupus erythematosus (SLE) compared to the general obstetric population. Hydroxychloroquine (HCQ), a recommended treatment to control disease activity during pregnancy, may reduce the risk of PTD. We and others reported that HCQ may reduce the risk of preeclampsia and hypertensive disorders of pregnancy. In our previous work with US claims data, over a third of pregnancies complicated by preeclampsia among patients with SLE resulted in PTD. We hypothesize that early pregnancy HCQ use in SLE pregnancy reduces the risk of PTD.
Methods: Data from all healthcare encounters, labs and pharmacy records were accessed from a large integrated health network in California from 2011-2020. SLE was defined as ≥2 coded visits ≥7 days apart (ICD9: 710.0, ICD10: M32.1, M32.9). Individuals with full pregnancy histories derived from the electronic health records were included and whose last menstrual period (LMP) date was after they satisfied the definition of SLE. We defined exposure as ≥2 HCQ fills 3 months pre-LMP through 1st trimester. Those with no fills during this time were considered unexposed. We estimated risk ratios (RR) and 95% confidence intervals (CI) for the association between HCQ use and PTD (< 37 gestational weeks) using propensity-score (PS) adjusted Poisson models to account for potential confounders such as maternal age, BMI, pregestational hypertension, history of lupus nephritis (LN), prepregnancy corticosteroids use, azathioprine use in pregnancy, antiphospholipid antibodies (aPL) status, and more. We investigated effect modification by aPL status, history of LN, pre-pregnancy hypertension, and corticosteroid use during pregnancy. Additionally, we used Cox models and treated PTD as a time to event analysis and estimated hazard ratios (HR) and 95% CIs. All analyses were stratified by parity (primipara vs multipara).
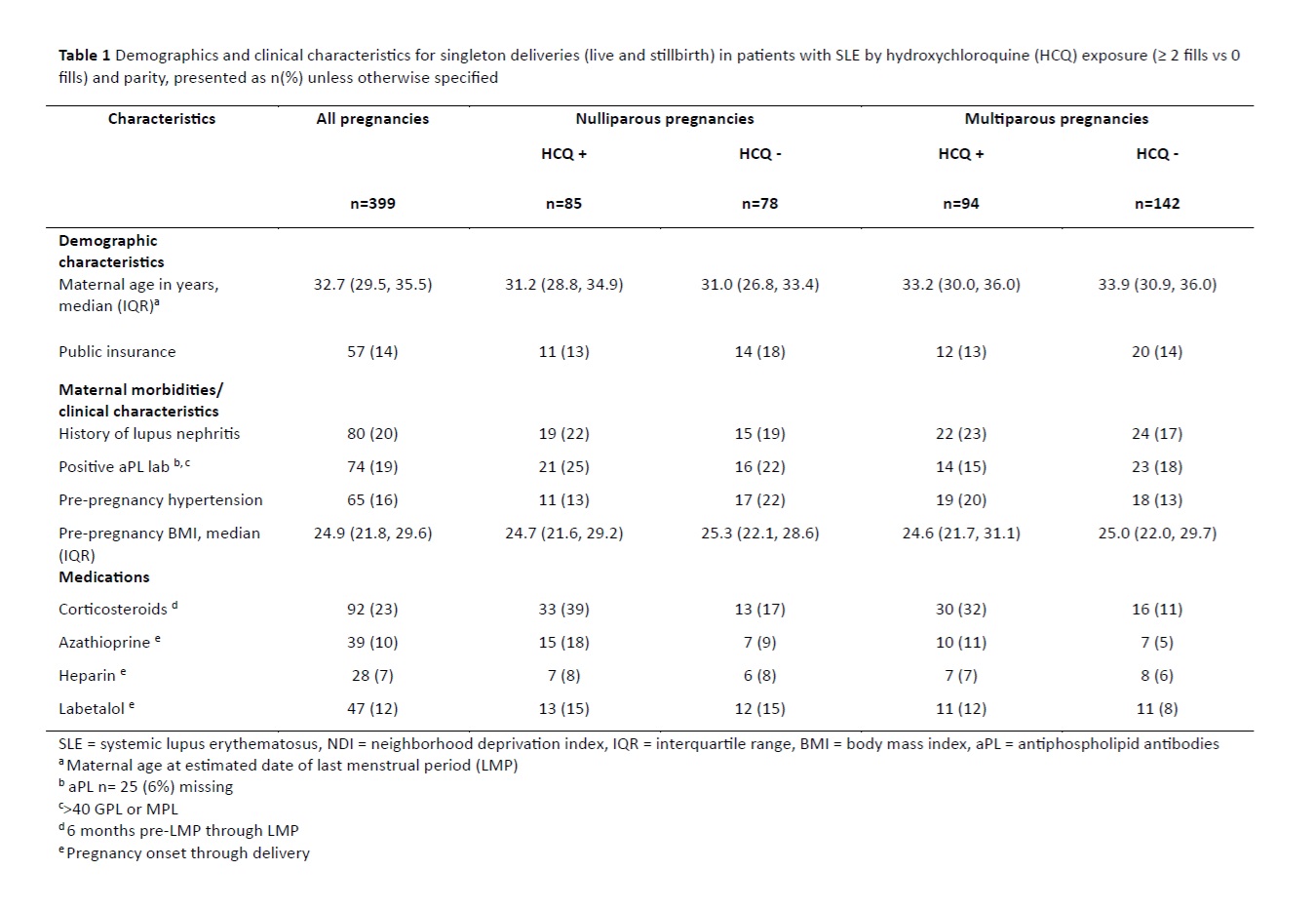
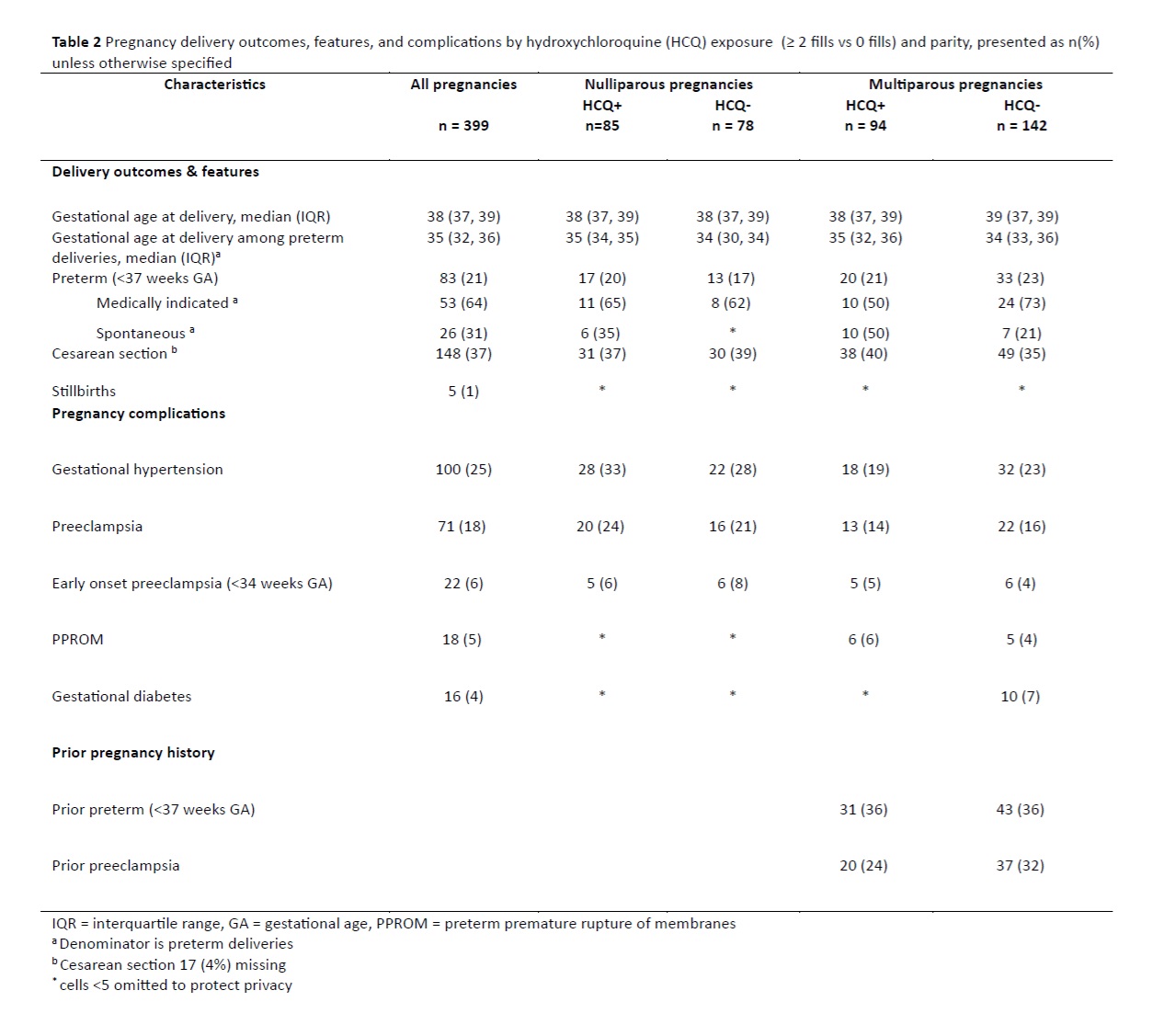
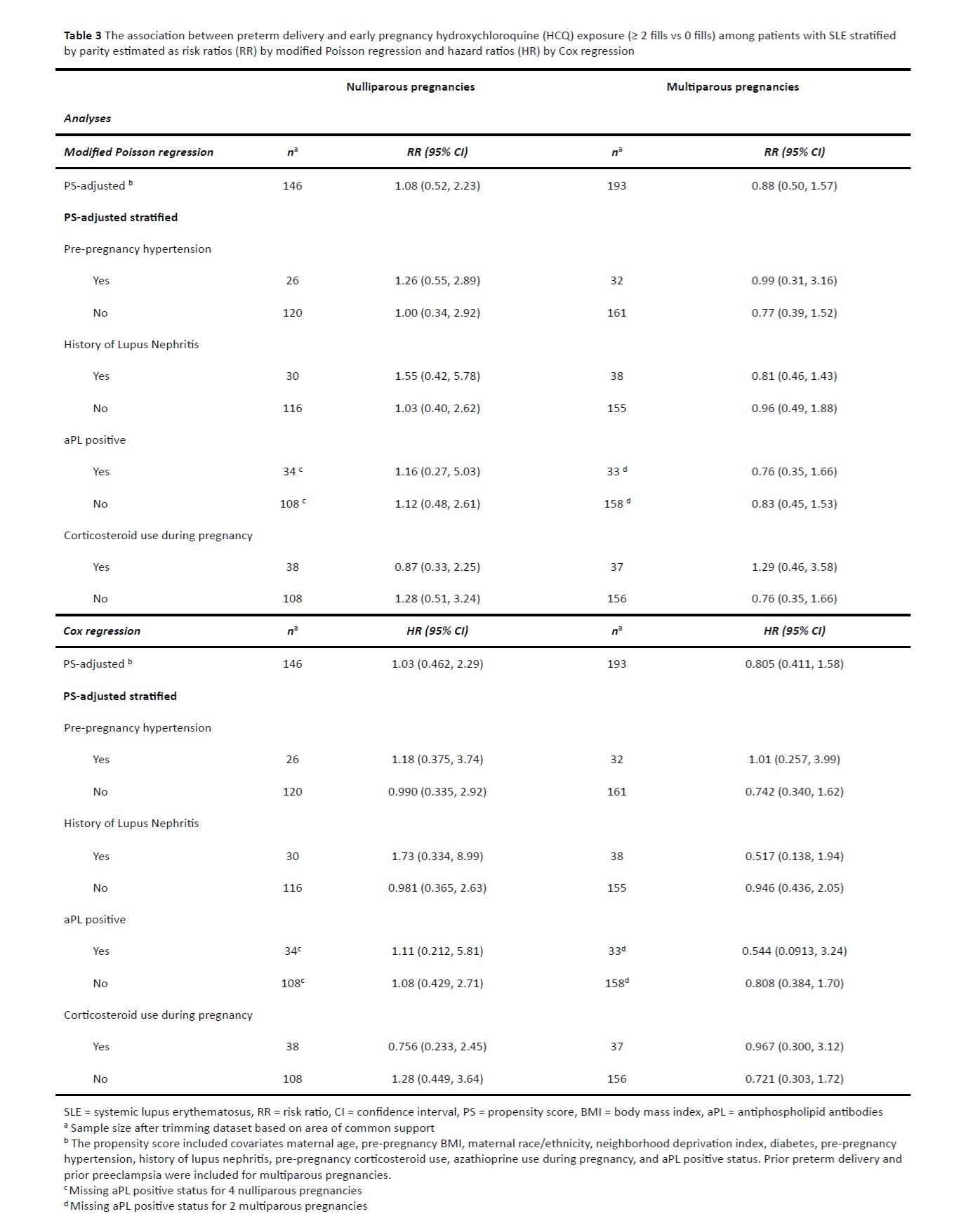
Results: There were 399 pregnancies among 324 racially and ethnically diverse patients with SLE. The nullipara pregnancies were more likely to be HCQ exposed (52% vs 40%). The HCQ exposed nullipara pregnancies had a higher prevalence of history of lupus nephritis and positive aPL, but less pregestational hypertension compared to the unexposed (13% vs 22%). Conversely, HCQ exposed multipara pregnancies had more pregestational hypertension compared to unexposed (20% vs 13%). (Table 1) Overall, 21% of pregnancies delivered preterm, of which 64% were medically indicated. The median gestational age among preterm deliveries was 35 weeks in the HCQ exposed and 34 weeks in the unexposed. (Table 2) The PS-adjusted RR was 1.08 (95% CI 0.52, 2.23) and 0.88 (95% CI 0.50, 1.57) among nulliparous and multiparous pregnancies, respectively. The PS-adjusted HR was 1.03 (0.46, 2.29) among nullipara, and 0.81 (0.41, 1.58) among the multipara pregnancies. Results did not differ appreciably in stratified analyses examining effect modification. (Table 3)
Conclusion: Use of HCQ early in SLE pregnancy was not associated with less PTD nor did it appreciably alter gestational age at delivery in multivariable-adjusted models.
To cite this abstract in AMA style:
Rector A, Liu E, Cantu M, Chakravarty E, Druzin M, Shaw G, Weisman M, Hedderson M, Simard J. Hydroxychloroquine Use in Early Pregnancy and Risk of Preterm Delivery in a Californian Cohort of Lupus Pregnancy [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-use-in-early-pregnancy-and-risk-of-preterm-delivery-in-a-californian-cohort-of-lupus-pregnancy/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-use-in-early-pregnancy-and-risk-of-preterm-delivery-in-a-californian-cohort-of-lupus-pregnancy/