Session Information
Date: Monday, November 9, 2020
Title: RA – Treatments II: Potential Harms & Adverse Events (1998–2002)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Hydroxychloroquine (HCQ) has been proposed as a potential treatment for COVID-19, but early reports suggested that it could have cardiotoxic effects. Despite widespread and longstanding use to treat rheumatic diseases, cardiotoxicity was not on the risk radar of most rheumatologists prescribing HCQ. Our objective in the present analysis is to examine the risk of cardiotoxicity among members of a well-characterized RA cohort who were receiving HCQ.
Methods: We studied RA patients meeting the 1987 ACR criteria, recruited from public, private, military, and Veterans Administration rheumatology clinics. Patients were invited to participate in yearly follow up evaluations in which we ascertained clinical and laboratory features, including HCQ use. The assessment included an electrocardiogram and a detailed medical record review.
We ascertained cardiac events, including myocardial infarction (MI), cardiomyopathy, cardiac conduction disorders, or cardiac dysrhythmias in all patients. We also ascertained vital status in all participants, obtaining a certificate for all deaths. We compared the frequency of these events between patients who received HCQ and patients who did not receive it using generalized estimating equation (GEE) models, including all follow-up visits. We considered HCQ exposure in three categories: Those who did not receive HCQ during the study period, those who were receiving it during some of the visits, and those who were receiving it at all of the visits. We adjusted for the propensity to receive HCQ to control for potential bias for indication.
Results: We studied 1328 patients, 981 of whom were women (74%). These patients completed 5826 visits, for a total of 8336 patient-years (pt-yrs) of follow up. There were 114 patients who were receiving HCQ at every one of 338 visits, for 347 pt-yrs; 323 patients who were receiving HCQ during some of 1742 visits, for 2793 pt-yrs; and 891 patients who were not receiving HCQ at any of 3746 visits, for 5147 pt-yrs. We ascertained 120 cases of MI, 185 of cardiac dysrhytmias, 13 cardiomyopathies, and 21 cases with conduction disorders. The table below shows the number and incidence of these events per 100 pt-yrs for each of the HCQ exposure categories.
We did not find significant differences in the risk of cardiomyopathy, conduction disorders, or MI between the three HCQ exposure categories. Cardiac dysrhythmias were significantly less likely to occur in patients who were taking HCQ compared to patients who were not taking it (OR 0.61, 95% CI 0.37-0.96, p= 0.03). The GEE regression models according to HCQ exposure, adjusted by age and sex, did not uncover associations between HCQ and cardiac events use (Table), nor did adding a score for the propensity to receive HCQ to the models. Patients who were receiving HCQ continuously had significantly lower mortality than those who were not receiving it at any of the visits (OR 0.55, 95% CI 0.40, 0.76, p < 0.0001).
Conclusion: Our findings suggest that HCQ is not associated with an increased risk of cardiotoxicity among RA patients. Moreover, HCQ exposure may be associated with a reduction in mortality among RA patients. HCQ appears to be safe among RA patients in terms of its cardiac effects.
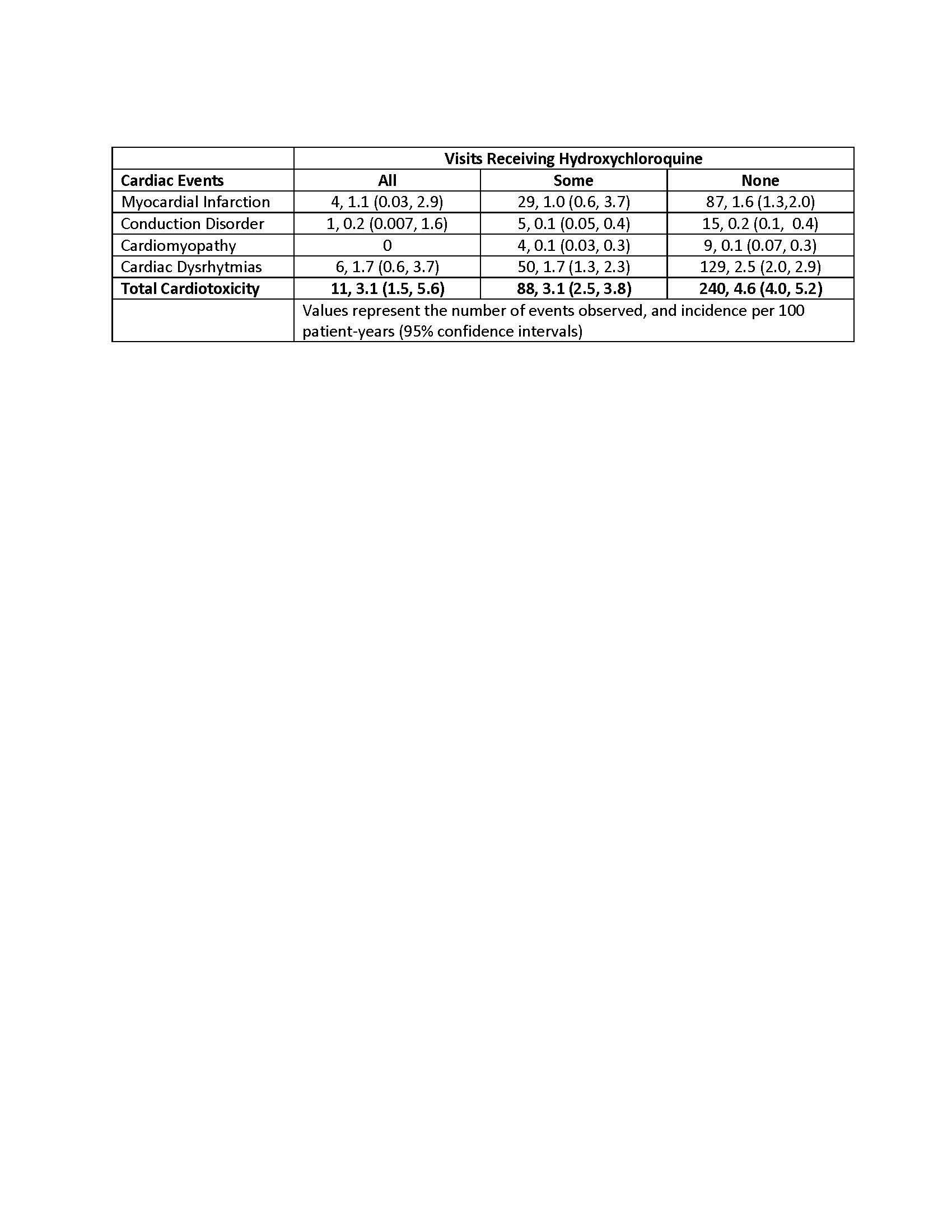 Table. Observed events and incidence of cardiotoxicity events according to HCQ exposure.
Table. Observed events and incidence of cardiotoxicity events according to HCQ exposure.
To cite this abstract in AMA style:
Restrepo J, Escalante A, Battafarano D, Lorenzo C, Del Rincon I. Hydroxychloroquine Is Not Cardiotoxic in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/hydroxychloroquine-is-not-cardiotoxic-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-is-not-cardiotoxic-in-patients-with-rheumatoid-arthritis/
