Session Information
Session Type: Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: The Strategy to Prevent the Onset of Clinically-Apparent Rheumatoid Arthritis (StopRA) (ClinicalTrials.gov NCT02603146) is a randomized, double-masked, placebo-controlled, multi-center (20 sites) clinical trial evaluating the efficacy and safety of hydroxychloroquine (HCQ) in the prevention of inflammatory arthritis (IA) and classifiable RA. The primary objective was to determine if treatment with HCQ for 1 year reduced the risk of developing IA and classifiable RA at the end of 3 years in individuals with elevated anti-cyclic citrullinated peptide antibodies (anti-CCP3, Werfen) and without IA at baseline. The results presented herein are from an interim analysis.
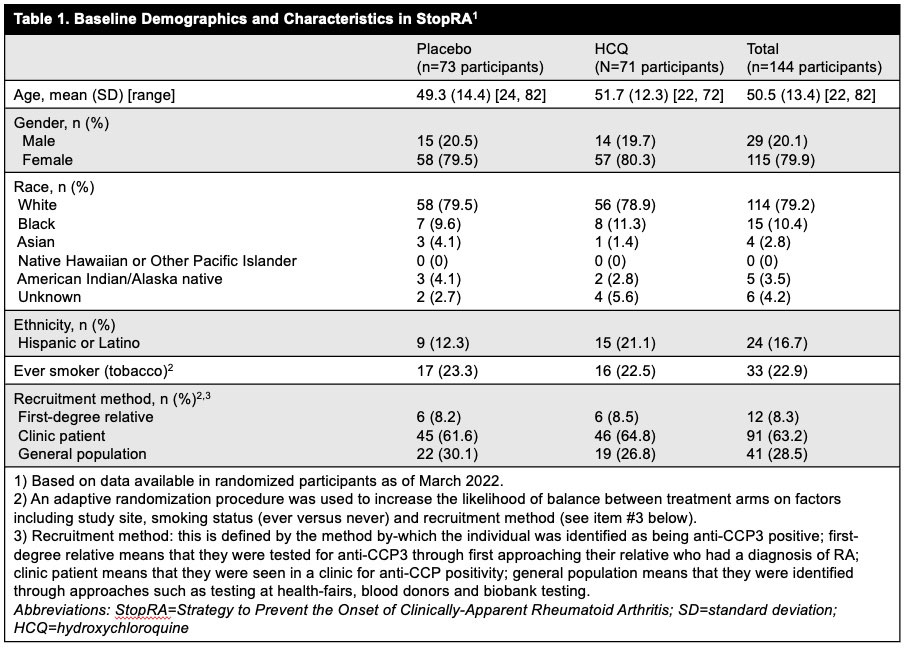
Methods: Primary inclusion criteria included adults (≥18) with anti-CCP3 ≥40 units, no history of IA or disease-modifying anti-rheumatic drug use, and absence at baseline of IA defined as a swollen joint consistent with RA-like synovitis on physical examination. The presence of joint symptoms/arthralgia and imaging findings were not used to determine eligibility. Participants were identified in rheumatology clinics and through de novo testing of first-degree relatives of patients with established RA, health-fair participants, blood donors and biobanks. Eligible participants were randomized 1:1 to receive HCQ (200-400 mg/day, weight-based) or placebo (PBO) for 1 year, with 2 years of post-drug follow-up. An adaptive randomization procedure was used to increase likelihood of balanced treatment arms (Table 1). For this interim analysis the primary efficacy endpoint was the development of IA classified as RA (score ≥6 by 2010 ACR/EULAR criteria), or IA and ≥1 erosion on x-ray. A modified intent-to-treat approach was used that included all eligible randomized participants who received at least 1 dose of study drug; risks for developing RA by 3 years in each arm were estimated with a Kaplan-Meier (KM) approach and compared using a Wald-type chi-square statistic. For this analysis, ~86% of data was available based on expectations from a completed trial, and criteria for efficacy and futility were pre-specified.
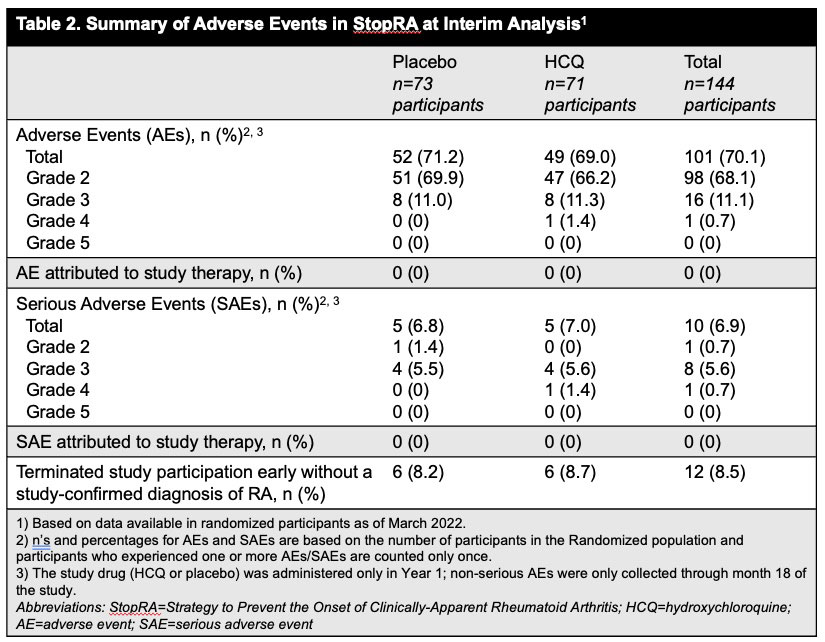
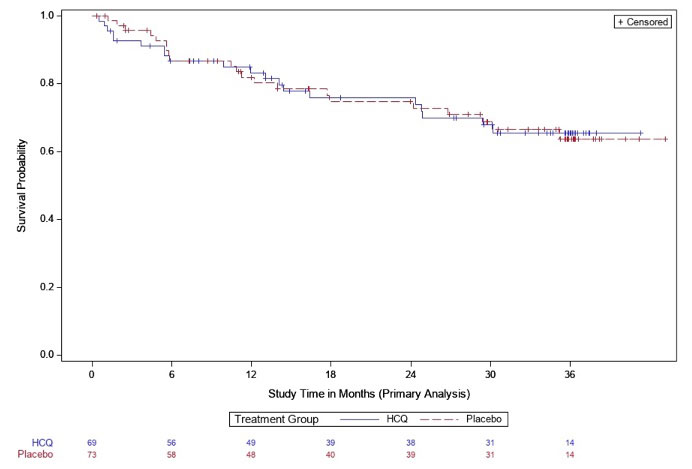
Results: Between Apr 2016 and Nov 2022 144 participants were randomized; 142 eligible participants who initiated study treatment (69 HCQ, 73 PBO) were included in the interim analysis. Demographics were balanced across groups (Table 1). At the time of interim analysis, 41 participants had developed RA and the KM-estimated probabilities of RA development were 34% in the HCQ arm and 36% in the PBO; p=0.844 (Figure 1). These findings met futility criteria (z score< 0.3). The overall rates of adverse events and early study terminations were similar between arms (Table 2).
Conclusion: These interim results from the StopRA trial demonstrate that, in individuals who are anti-CCP3(+) without IA at baseline, 1 year of HCQ is not superior to placebo in preventing or delaying the development of IA and classified RA at 3 years. Thus, the study was halted due to futility. Of note, the study accrued 41 individuals who developed RA, and KM estimates suggest the rate of conversion to RA is ~35% by 3 years; these findings support the use of an anti-CCP3 ≥40 criterion in future studies designed to identify interventions to prevent or delay IA and classifiable RA.
To cite this abstract in AMA style:
Deane K, Striebich C, Feser M, Demoruelle K, Moss L, Bemis E, Frazer-Abel A, Fleischer C, Sparks J, Solow E, James J, Guthridge J, Davis J, Graf J, Kay J, Danila M, Bridges, Jr. S, Forbess L, O'Dell J, McMahon M, Grossman J, Horowitz D, Tiliakos A, Schiopu E, Fox D, Carlin J, Arriens C, Bykerk V, Jan R, Pioro M, Husni M, Fernandez-Pokorny A, Walker S, Booher S, Greenleaf M, Byron M, Keyes-Elstein L, Goldmuntz E, Holers V. Hydroxychloroquine Does Not Prevent the Future Development of Rheumatoid Arthritis in a Population with Baseline High Levels of Antibodies to Citrullinated Protein Antigens and Absence of Inflammatory Arthritis: Interim Analysis of the StopRA Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-does-not-prevent-the-future-development-of-rheumatoid-arthritis-in-a-population-with-baseline-high-levels-of-antibodies-to-citrullinated-protein-antigens-and-absence-of-inflammatory/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-does-not-prevent-the-future-development-of-rheumatoid-arthritis-in-a-population-with-baseline-high-levels-of-antibodies-to-citrullinated-protein-antigens-and-absence-of-inflammatory/