Session Information
Date: Monday, November 9, 2020
Title: SLE – Treatment Poster II
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Hydroxychloroquine (HCQ) and vitamin D are both immunomodulators in SLE, but work through different mechanisms. Hydroxychloroquine has been proven to triple renal response to mycophenolate in membranous nephritis (Lupus 2006;15: 366–70) and may reduce end stage renal disease (Arthritis Rheum 2009;61:830–39). Vitamin D reduces proteinuria in SLE (Arthritis Rheum 2013;65:1865–71) and in other renal diseases as well. We asked whether these effects were synergistic or additive.
Methods: SLE patients met revised ACR or SLICC classification criteria: 332 patients with biopsy-proven lupus nephritis (89% female, 54% African-American, 28% Caucasian) also had subsequent measurements of HCQ blood levels (method of Füzéry et al. Clin Chim Acta 2013;421:79–84) at the visits. After removing visits that had a large gap of more than 1 year, there were 324 patients with 3,444 visits. The number of HCQ blood level measures per patient ranged from 1 to 34 with a median of 11 measures. Generalized estimating equations (GEE) were used to look at the relationship between proteinuria (by urine pr/cr) and HCQ blood levels at the same visit, accounting for repeated measures in the same person. A conditional logistic regression was used to perform a “within-person” analysis to look at the renal outcomes more closely. In this analysis, each person served as his/her own control, by comparing visits with increased proteinuria to visits with less proteinuria with respect to mean HCQ blood level.
Results: There were no significant differences between mean HCQ blood levels and sex (p=0.7166), ethnicity (p=0.5402), or mean body mass index (r=-0.02, p=0.6970), but significant results were observed for age (r=0.19, p=0.006). Using GEE models which account for repeated measures from the same person, the effects of vitamin D and of HCQ blood level on proteinuria were still significant after adjusting for each other (Table 3). However, the effect of HCQ blood level diminished (borderline significance) after further adjustment for sex, age, and race. Vitamin D appeared to be the stronger predictor of proteinuria. Because the probability of proteinuria changed for HCQ levels when vitamin D level was below or above approximately 20 ng/ml, we looked at the effects of HCQ blood levels on proteinuria stratifying by vitamin D levels. Results were adjusted for repeated measures in patients. There was no evidence of an association between HCQ blood levels and proteinuria if vitamin D was below 20 (OR 1.02, 95% CI 0.98, 1.07). When vitamin D levels were >=20, there was a reduced risk of proteinuria with higher HCQ blood levels (OR 0.97, 95% CI 0.95, 0.99, p=0.009).
Conclusion: Although both HCQ and vitamin D reduce proteinuria, in multivariate GEE models, vitamin D had the more important benefit. When vitamin D levels were >= 20 ng/mL, there was reduced proteinuria with higher HCQ blood levels. In clinical practice and in randomized clinical trials, prescribed HCQ and vitamin D (as well as adherence to them) will benefit proteinuria and thus improve renal response.
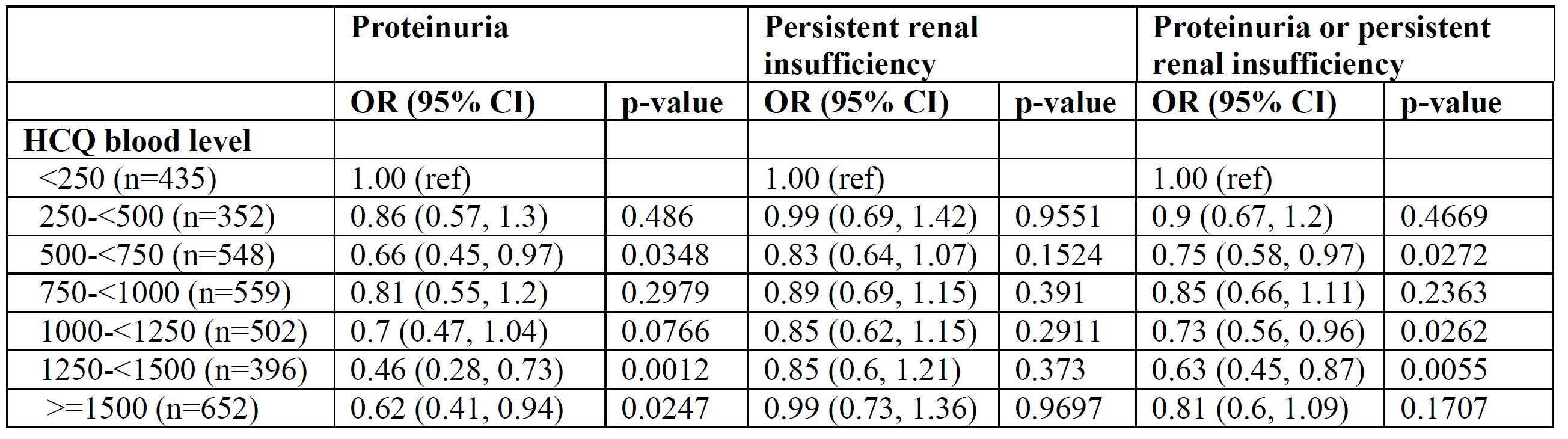 Adjusted associations between renal outcomes and HCQ Levels, adjusting for covariates.
Adjusted associations between renal outcomes and HCQ Levels, adjusting for covariates.
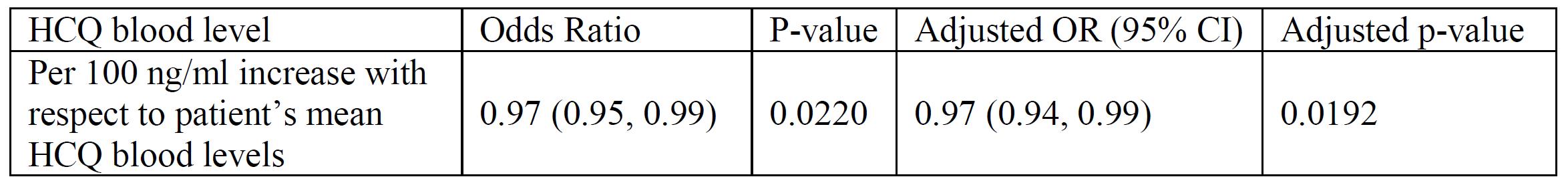 Within-person analysis of the relationship between proteinuria and HCQ blood levels: unadjusted and adjusted (adjusted for age, BMI, Cellcept, Imuran, ACE-inhibitor or ARB use, and implicitly for all time-invariant characteristics within a person)
Within-person analysis of the relationship between proteinuria and HCQ blood levels: unadjusted and adjusted (adjusted for age, BMI, Cellcept, Imuran, ACE-inhibitor or ARB use, and implicitly for all time-invariant characteristics within a person)
 Effect of vitamin D and hydroxychloroquine on proteinuria, within person analysis using GEE.
Effect of vitamin D and hydroxychloroquine on proteinuria, within person analysis using GEE.
To cite this abstract in AMA style:
Petri M, Li J, Goldman D. Hydroxychloroquine and Vitamin D Both Reduce Proteinuria in SLE [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/hydroxychloroquine-and-vitamin-d-both-reduce-proteinuria-in-sle/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-and-vitamin-d-both-reduce-proteinuria-in-sle/
