Session Information
Date: Tuesday, October 28, 2025
Title: (2195–2226) Reproductive Issues in Rheumatic Disorders Posters
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
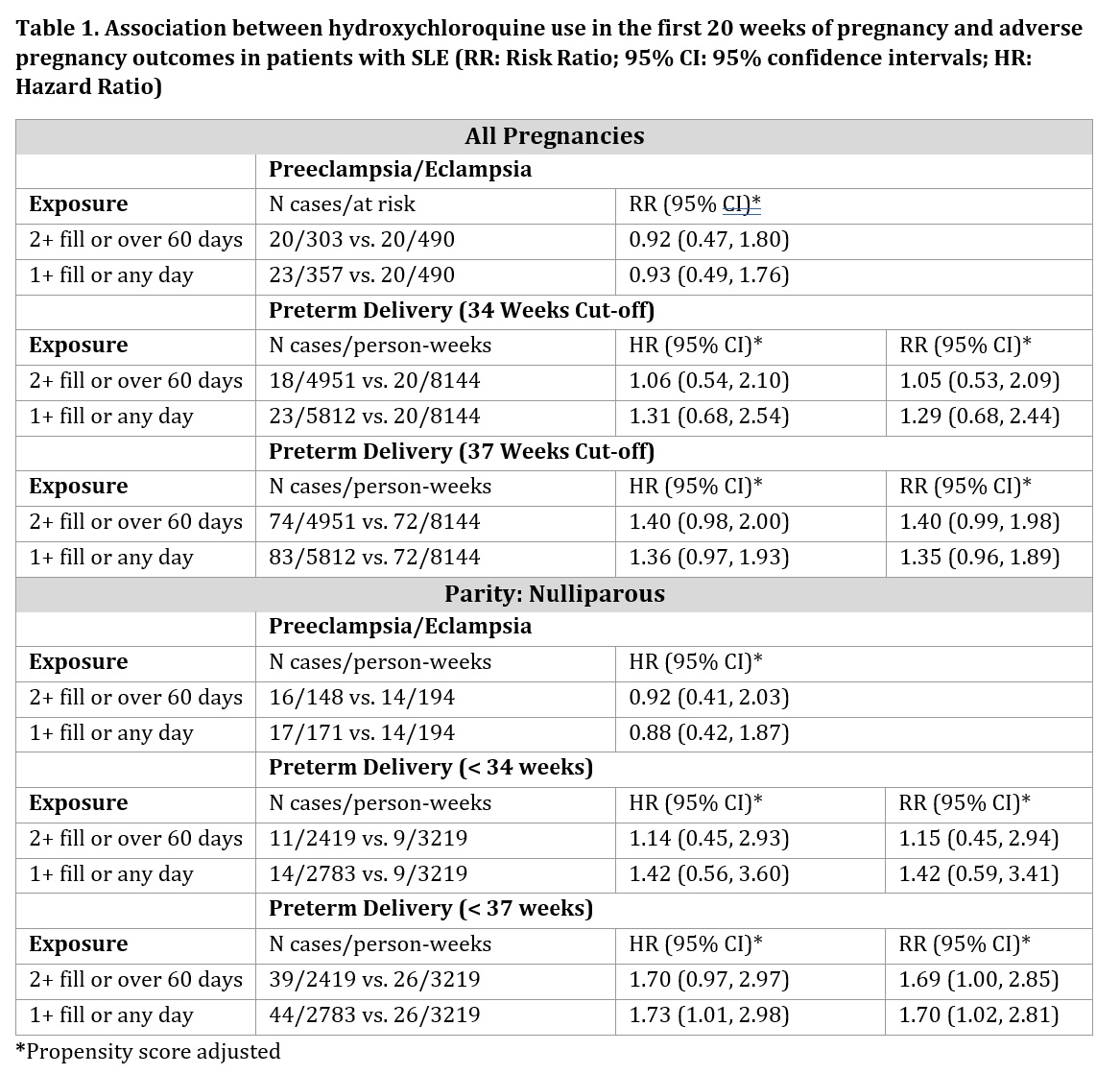
Background/Purpose: Systemic lupus erythematosus (SLE) can pose an increased risk of adverse pregnancy outcomes such as preeclampsia and preterm delivery. Hydroxychloroquine (HCQ), commonly used to manage SLE, especially during pregnancy, has shown conflicting associations with pregnancy outcomes, highlighting an important knowledge gap. In this study, we explored the association between HCQ use and the risk of preeclampsia/eclampsia and preterm delivery, while accounting for important maternal covariates in a cohort of SLE pregnant patients.
Methods: We studied SLE singleton pregnancies among publicly insured patients in the British Columbia Perinatal Data Registry (BCPDR). We studied pregnancies where SLE was recorded before pregnancy and defined HCQ treatment during pregnancy multiple ways, including at least two fills or minimum of 60 days’ supply during the first 20 weeks of pregnancy. For binary outcomes modified Poisson models estimated risk ratios (RRs) and 95% confidence intervals (CIs) using propensity scores to address confounders. For preterm delivery, we also conducted a time-to-event analysis using a Cox proportional hazards model. Multiple sensitivity analyses explored definitions of SLE, HCQ exposure, and outcomes and stratified analyses examined effect modification.
Results: We studied 847 pregnancies to 597 women with SLE during pregnancy. Most pregnancies were among non-smokers at the start of pregnancy (93.9%). The mean age was 33 years old (SD=4.6 years). Use of HCQ, defined as ≥2 fills or ≥60 days’ supply compared to no fills, had an adjusted RR of 0.92 (95% CI: 0.47, 1.80) for preeclampsia/eclampsia overall. Results were similar when expanding the exposure window to include three months preconception. In sensitivity analyses, the RR for the early-onset phenotype was smaller (0.72 CI: 0.27, 1.92) although based on smaller numbers. For preterm delivery, we found generally no association across different definitions and approaches. For example, ≥2 fills had an adjusted hazard ratio (HR) of 1.06 (95% CI: 0.54, 2.10). The stratified analyses results showed no appreciable differences.
Conclusion: HCQ use during early pregnancy was not significantly associated with the risk of preeclampsia or preterm delivery. Like past work, the RR was below the null for preeclampsia, but not statistically significant. Residual confounding and misclassification cannot be excluded, and further larger studies with detailed clinical data may shed light on these findings as the potential impact of HCQ on pregnancy outcomes is needed.
To cite this abstract in AMA style:
Sediqi S, lu l, Aviña-Zubieta J, Simard J. Hydroxychloroquine and Pregnancy Outcomes in Lupus: Results from a population-based cohort study [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-and-pregnancy-outcomes-in-lupus-results-from-a-population-based-cohort-study/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-and-pregnancy-outcomes-in-lupus-results-from-a-population-based-cohort-study/