Session Information
Date: Sunday, November 17, 2024
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: The current treatment guidelines for systemic lupus erythematosus emphasize the universal use of antimalarials such as hydroxychloroquine (HCQ) unless contraindicated as well as limited exposure to corticosteroids if possible. Adherence to these guidelines may then represent benchmarks of quality of care that can be targeted by individual providers, practices, and health systems. This study was undertaken to learn the rate of prescription and extent of the use of HCQ by individuals diagnosed with SLE, as well as their use of varying doses of prednisone (PRD) over a five-year period from 2019-2024.
Methods: The TriNetX Research Network contains continuously updated, deidentified information from the electronic health records (EHR) of 126.9 million individuals in 89 healthcare organizations. A dataset consisting of the records of 113,041 individuals who had their first (index) appearance of a systemic lupus erythematosus diagnostic code (M32.*) on or after 1/1/19 was obtained, and individuals were placed into cohorts based on the number of subsequent appearances of M32.*, with at least two instances in the first 360 days representing a ‘confident’ diagnosis. The records were searched for the appearance of prescriptions for HCQ and PRD both before and after the index diagnosis.
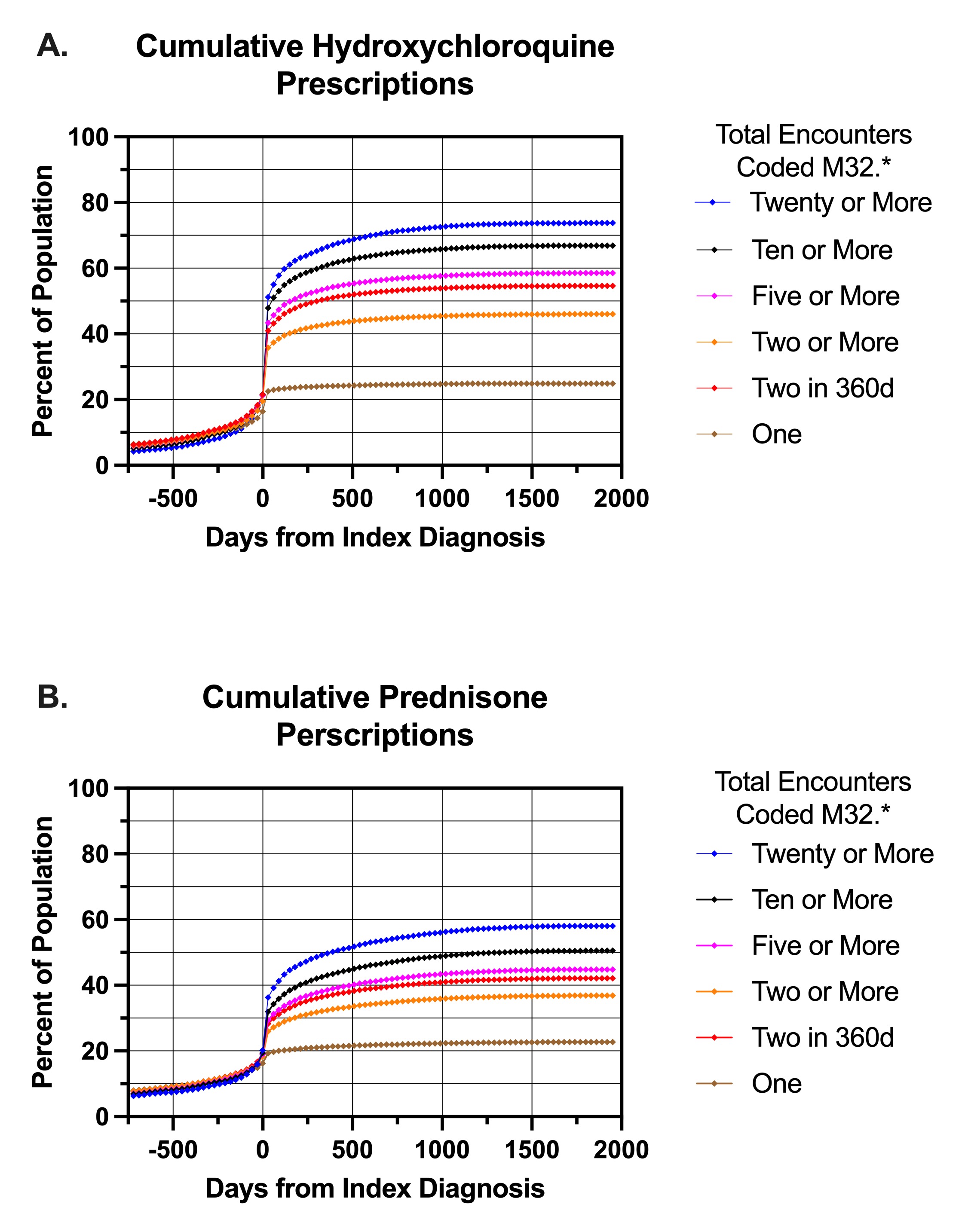
Results: The cumulative appearance of ambulatory HCQ prescriptions in the EHR over time is shown in panel A for individuals who had ‘confident’ lupus diagnoses (n=34,683) as well as 1 (least confident, n=22,598), ≥2 (n=52,561), ≥5 (n=27,102), ≥10 (n=15,608), and ≥20 (n=7,687) instances of M32.* coded encounters in the period 1/1/19-4/19/24. The greatest association of HCQ with M32.* diagnoses occurred in individuals who had >20 visits for that diagnosis, which would be typical for lupus patients (~4 visits/year). HCQ prescriptions increased to ~50% in this group just after the index diagnosis, and continued to rise to 73% over the next 1000 days . Panel B shows the appearance of any ambulatory PRD prescription for the same cohorts. Again, PRD use of any duration increased rapidly after diagnosis and 56% of the most frequently seen SLE patients got at least one dose of PRD. 21.5% of patients received a prescription for 10mg or larger tablets of PRD. Interestingly, we noted a measurable use of both HCQ and PRD in individuals before their index diagnosis of lupus.
Conclusion: Using a large EHR database of individuals diagnosed as having SLE, the provision of HCQ occurs rapidly after diagnosis but takes nearly 3 years to reach over 70% in patients with the most lupus encounters. PRD was given to over 40% of patients with a ‘confident’ diagnosis of lupus and half of these people were prescribed prednisone in sizes resulting in >5mg/d. While this study is limited by the use of diagnostic codes to define lupus, it represents ‘real world’ data and suggests an area for quality improvement. The appearance of HCQ and PRD before the index diagnosis may represent ‘confident’ diagnoses outside the TriNetX HCO network, or may be the result of treatment for earlier stages of lupus not diagnosed as such.
To cite this abstract in AMA style:
Narvaez M, Basit M, Karp D. Hydroxychloroquine and Prednisone Use by Individuals Diagnosed with Systemic Lupus Erythematosus in the TriNetX Research Database [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-and-prednisone-use-by-individuals-diagnosed-with-systemic-lupus-erythematosus-in-the-trinetx-research-database/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-and-prednisone-use-by-individuals-diagnosed-with-systemic-lupus-erythematosus-in-the-trinetx-research-database/