Session Information
Date: Monday, October 27, 2025
Title: (1467–1516) Systemic Lupus Erythematosus – Diagnosis, Manifestations, & Outcomes Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Patients with systemic lupus erythematosus (SLE) are at increased risk for herpes zoster (HZ) and its complications. Exposure to immunosuppressants (IS) at the time of the 2-dose recombinant zoster vaccine (RZV) may attenuate responses. We evaluated humoral immune responses to the RZV in a longitudinal SLE cohort, including individuals exposed and unexposed to IS.
Methods: Cohort participants meet ACR SLE criteria and have clinical data and biospecimens collected annually. We selected participants who received ≥1 RZV dose according to the provincial registry (2018-2024) and had available serum samples before vaccination plus at ≥1 time point after the first and/or second RZV dose. We determined serum anti-Varicella Zoster Virus glycoprotein E antibody (anti-gE Ab) concentrations using an enzyme-linked immunosorbent assay (ELISA, ReVacc Scientific, USA). We defined the humoral response threshold as a ≥4-fold increase in anti-gE Ab concentration compared to pre-vaccination titres. We determined the proportion of RZV recipients above the response threshold (RZV response rate), compared RZV responses according to IS exposure, and evaluated the persistence of humoral responses over time.
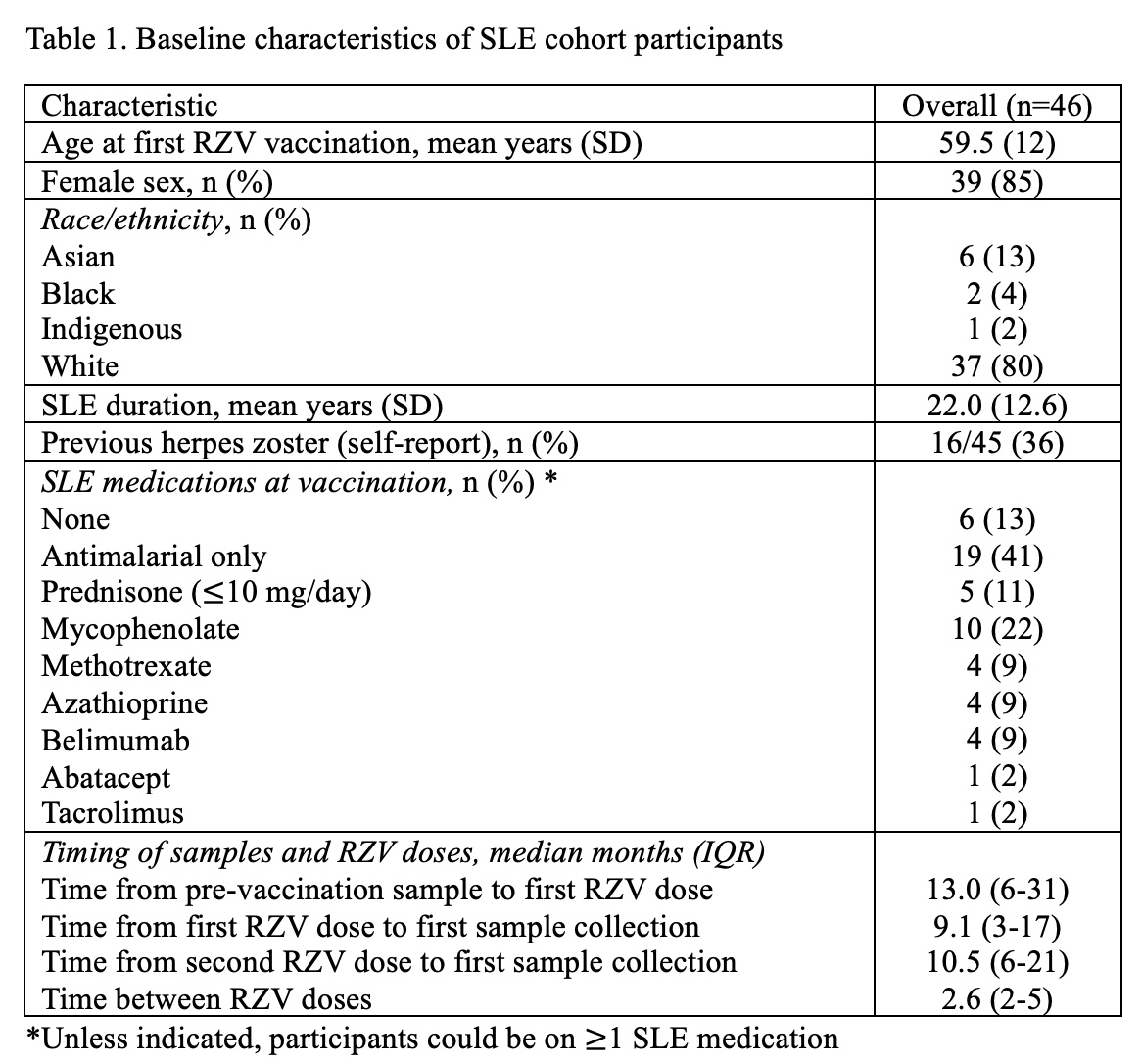
Results: We tested baseline and post-vaccine samples from 46 SLE cohort participants, 9 with samples collected after one RZV dose and 40 with samples collected after two doses (Table 1). All were seropositive for anti-gE Ab at baseline and 45/46 (98%) had an increase in anti-gE Ab concentrations following ≥1 RZV doses. The RZV response threshold was met in 5/9 (56%) at a median of 9 months (IQR 3-17) from the first RZV dose, and in 26/40 (65%) at a median of 11 months (IQR 6-21) from the second dose (mean ± SD anti-gE Ab concentration 27291 ± 29949 ng/ml post-first dose and 46471 ± 36933 ng/ml post-second dose, compared to 6341 ± 9270 ng/ml at baseline, Figure 1). Response rates to two doses were similar in those unexposed (15/22, 68%) and exposed to IS (11/18, 61%) at the time of vaccination, and in those who received 2 RZV doses ≤65 days apart (8/12, 67%) versus >65 days apart (18/28, 64%). Anti-gE Ab concentrations decreased according to time since vaccination (Figure 2), and in samples drawn ≥3 years post-vaccination, mean (±SD) anti-gE Ab concentration was not significantly different from baseline (15775 ± 9562 ng/ml versus 6417 ± 9184 ng/ml).
Conclusion: We detected a humoral response in most (68%) patients with SLE who received 2 doses of RZV, regardless of background treatment. However, increases in anti-gE Ab appear to return to baseline concentrations ≥3 years post vaccination.
.jpg) Figure 1. Anti-gE Ab concentrations (ng/mL) pre-vaccination, and after first and second RZV dose
Figure 1. Anti-gE Ab concentrations (ng/mL) pre-vaccination, and after first and second RZV dose
.jpg) Figure 2. Anti-gE Ab concentrations (ng/mL) at baseline and over time after the 2-dose RZV
Figure 2. Anti-gE Ab concentrations (ng/mL) at baseline and over time after the 2-dose RZV
To cite this abstract in AMA style:
Mendel A, Lora M, Bernatsky S, Fortin P, Desjardins M, Rauch J, Sauvageau C, Vinet E, Colmegna I. Humoral immune responses to real-world recombinant zoster vaccination in systemic lupus erythematosus [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/humoral-immune-responses-to-real-world-recombinant-zoster-vaccination-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/humoral-immune-responses-to-real-world-recombinant-zoster-vaccination-in-systemic-lupus-erythematosus/