Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: To prevent infections, EULAR recommends to vaccinate RA patients against streptococcal pneumoniae. PCV13 is a T- cell dependent vaccine whereas PPSV23 induces a T-independent humoral response. Abatacept is a biological DMARD that inhibits T cell activation. We hypothesized that the humoral response after PCV13 vaccination may be more affected under abatacept than after PPSV23. To answer this question, we compared the humoral response 1, 6 and 12 months after vaccination with PCV13/PPSV23 or PPSV23 in RA patients starting abatacept
Methods: We conducted a prospective, multicentre, randomized, open-label study in patients with RA according to ACR/EULAR 2010 criteria, active (DAS28 >3,2) under MTX or leflunomide and proposed to start abatacept. Patients were randomized in 2 groups: the first group (G1) was vaccinated with the PPSV23 and the second group (G2) with PCV13 and PPSV23 8 weeks later. Abatacept was initiated at the same time as vaccination. Patients previously treated with rituximab within the last year were excluded. To measure humoral response, serum was collected 4 weeks after the first vaccine and at 6 and 12 months. A patient was considered as responder if there was a two-fold increase of the antibodies titer for at least 3 of 5 serotypes of interest (1, 3, 14, 7F, 19A) which are the most frequently involved in pneumococcal infections. For the primary endpoint, we compared the rate of responders at one month in G1 and G2 using a Chi-Square test (alpha risk 5% and 80% power). For the secondary end points, we compared humoral response at 6 and 12 months between G1 and G2. A logistic regression was used to identify factors associated with the humoral response at 1, 6 and 12 months. Tolerance of the vaccines (injection site reaction and general reaction) was recorded within the first week and later in a patient booklet. Serious adverse events (SAEs) and related to study participation or study vaccine, were recorded at 1, 6 and 12 months.
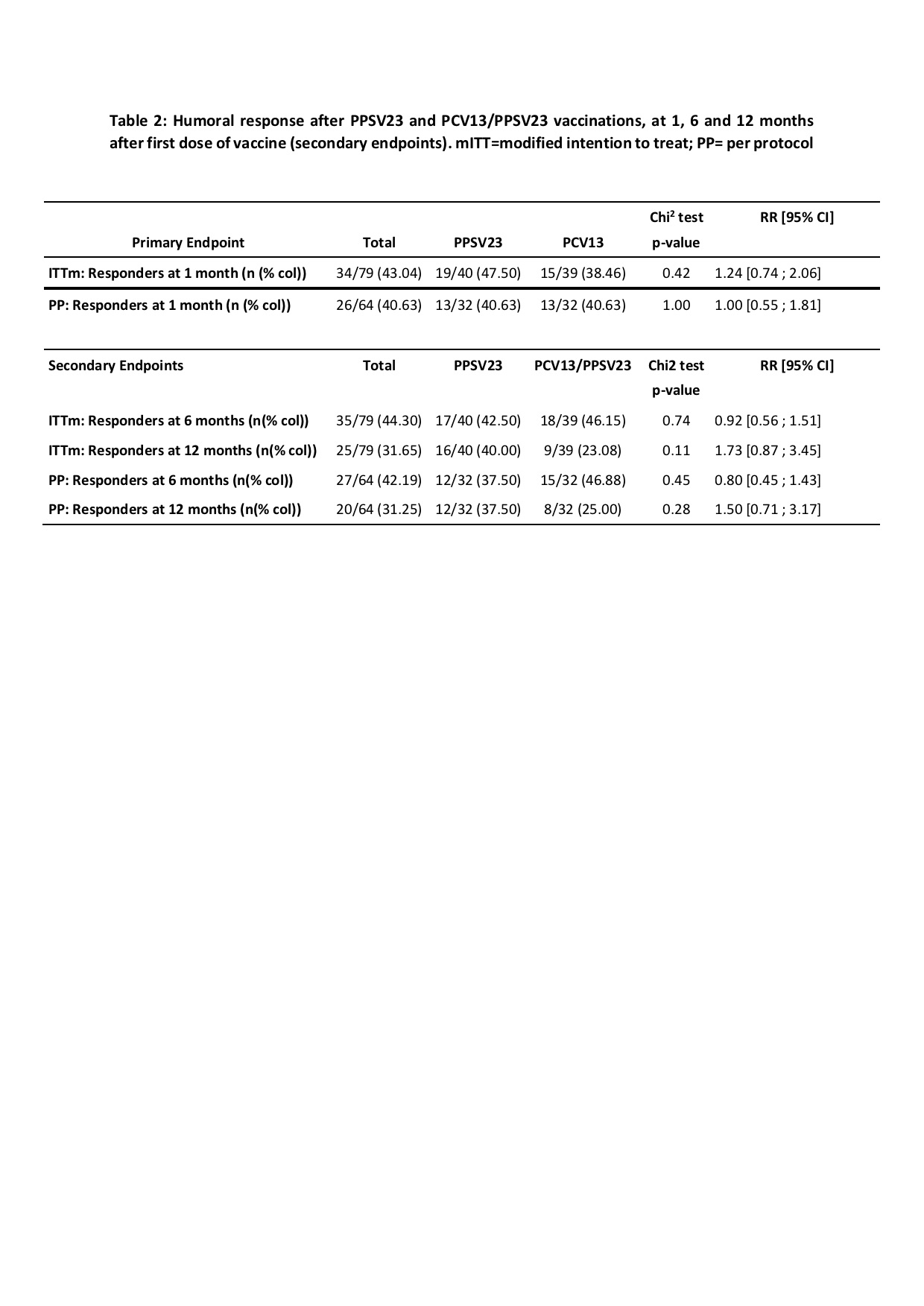
Results: Eighty patients were included and randomized in the two groups: 40 in G1 and 39 in G2 because of consent withdrawal for one patient. Characteristics of patients are described in table 1. Female were more represented in G1 (82.5%) compared to G2 (64.1%). Lymphocyte count was significantly higher in G1 compared to G2: 1841/mm3 (+/-887) vs 1603/mm3 (+/-580). In the mITT, the rate of responders was 47.5% in in G1 and 38.46% in G2 with a RR of 1.23 (IC95%: 0.73-2,06) when comparing responders in PPSV23 vs PCV13 groups (table 2). At 6 and 12 months, there was no difference between G1 and G2 in mITT and PP analysis. Logistic regression did not identify factors associated with humoral response at 1 and 6 months. At 12 months, gender (being a male vs female) and PPSV23 vs PCV13/PPSV23 were associated with a better response: OR 3,7 [IC95% 1.2-12] and 3,1 [IC95% 1-9.4] respectively (p< 0.05) . 17 infections were reported in G1 and 28 in G2 with 3 severe infections but no pneumococcal infection.
Conclusion: In RA patients treated with abatacept combined with csDMARDs (MTX or LEF), the rate of responders is similar at 1-, 6- and 12-months following vaccination with PCV13 or PPSV23.Abatacept does not seem to impact humoral response of PCV13 in the first 6 months following vaccination.
To cite this abstract in AMA style:
MOREL J, Brocq O, Gaujoux Viala C, Constantin A, Lassoued S, Dernis E, Richez C, Lukas C, Daien C, Duflos C. Humoral Immune Response to 13 Valent-conjugate and 23-valent Polysaccharide Pneumococcal Vaccines in RA Patients Treated with Abatacept: Results of the Open Randomized Controlled Trial VACINA (Vaccination Against Pneumococcal in Naïve Abatacept Rheumatoid Arthritis Patients) [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/humoral-immune-response-to-13-valent-conjugate-and-23-valent-polysaccharide-pneumococcal-vaccines-in-ra-patients-treated-with-abatacept-results-of-the-open-randomized-controlled-trial-vacina-vaccina/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/humoral-immune-response-to-13-valent-conjugate-and-23-valent-polysaccharide-pneumococcal-vaccines-in-ra-patients-treated-with-abatacept-results-of-the-open-randomized-controlled-trial-vacina-vaccina/