Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Patients with immune mediated diseases achieve lower seroconversion rates to COVID19 vaccines compared to healthy controls, which is why these patients are a prioritized group to receive a third dose as part of their primary regimen. The aim of this study was to assess the SARS-CoV-2-specific humoral and T-cell responses after a two-dose regimen of SARS-CoV-2 vaccine in patients with rheumatoid arthritis (RA) and after a third dose in those with undetectable antibodies titles after the primary regimen.
Methods: Observational study. Patients with RA (ACR/EULAR 2010 criteria), ≥18 years old, who were vaccinated according to the vaccination strategy of the Argentinean National Health Ministry were included. Anti-SARS-CoV-2 IgG antibodies (ELISA-COVIDAR test), neutralizing activity and specific T-cell responses (IFN-γ ELISpot Assay) were assessed between 21 and 40 days after the first and second doses in all patients and after the third dose in those with no seroconversion after two doses. Statistical analysis: descriptive analysis. Chi2 or Fischer test and T test. Multiple logistic regression models.
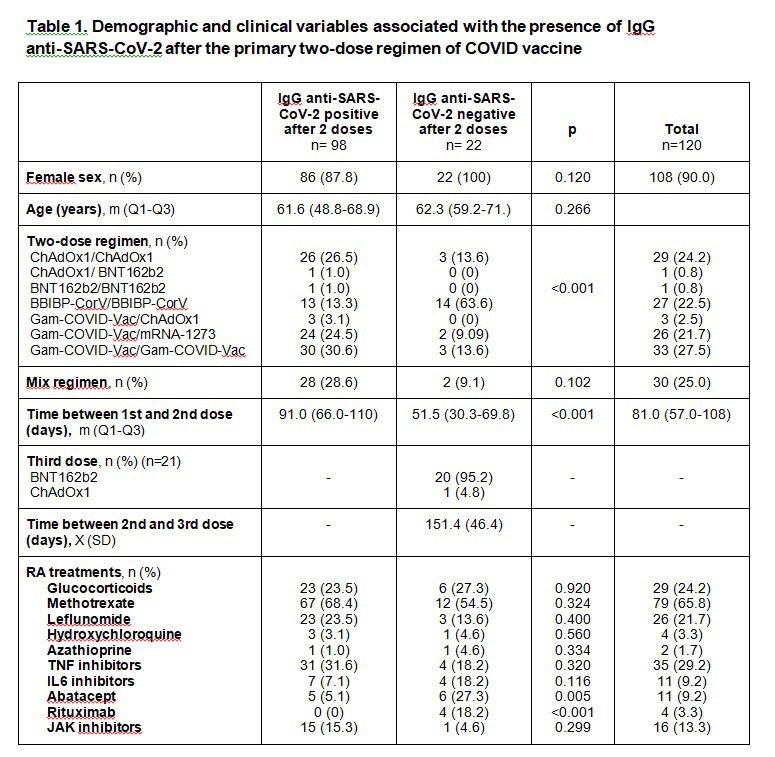
Results: A total of 120 RA patients were included, 90% were females with a median age of 61.6 years (Q1,Q3 50.2,69.6) and mean disease duration of 13.2 years (SD 8). History of SARS-CoV-2 infection was reported in 7 (5.8%) patients. Vaccine regimens and RA treatments are described in Table 1. After the second dose 81.7% presented anti-SARS-CoV-2 antibodies, 65% neutralizing activity (median titer 1/32, Q1-Q3 1/4-1/128) and 83% specific T-cell response. The use of BBIBP-CorV, treatment with Abatacept (ABT) and Rituximab (RTX) were associated with undetectable antibodies and no neutralizing activity after two doses of vaccine. BBIBP-CorV was also associated with the absence of T-cell response. The total incidence of adverse events was 357.1 events/1000 doses; significantly lower with BBIBP-CorV (166.7 events/1000 doses, p< 0.02). Five patients (4.2%) reported a disease flare. A third COVID vaccine dose was given to 21 patients with undetectable antibodies after the primary regimen (one patient was vaccinated outside the protocol), 90.5% of the patients presented detectable anti-SARS-CoV-2 IgG and 76.2% neutralizing activity after the third dose. Compared to other treatments, ABT and RTX were associated with no neutralizing activity. Additionally, the use of ABT was associated with a lower frequency of T-cell response.
Conclusion: In this RA cohort, 81.7% presented anti-SARS-CoV-2 antibodies after two doses of SARS-CoV-2 vaccine. The use of BBIBP-CorV, ABT and RTX was associated with lower frequency of humoral response, while BBIBP-CorV was also associated with the absence of T-cell response. Within those who failed to seroconvert after two doses, 90.5% presented detectable anti-SARS-CoV-2 IgG after a third dose. This data highlights the importance of additional vaccine doses in this group of patients.
To cite this abstract in AMA style:
Isnardi C, Cerda O, LANDI M, Cruces L, Schneeberger E, Calle montoro C, Alfaro M, roldan B, Gomez Vara A, Giorgis P, Ezquer R, Crespo Rocha M, Reyes Gómez C, correa m, Rosemffet M, Carrizo Abarza V, Catalan Pellet S, Perandones M, Reimundes C, Longueira Y, Turk G, Quiroga M, Laufer N, Quintana R, De la Vega M, Kreplak N, Pifano M, Maid P, Pons-Estel G, Citera G. Humoral and T-cell Responses to SARS-CoV-2 Vaccination in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/humoral-and-t-cell-responses-to-sars-cov-2-vaccination-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/humoral-and-t-cell-responses-to-sars-cov-2-vaccination-in-patients-with-rheumatoid-arthritis/