Session Information
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Bimekizumab (BKZ) is a monoclonal IgG1 antibody that selectively inhibits interleukin (IL)-17F in addition to IL-17A. Two phase 3 studies were conducted where treatment with BKZ demonstrated efficacy and was shown to be well-tolerated to 52 weeks across the full disease spectrum of axial spondyloarthritis (axSpA): BE MOBILE 1 (non-radiographic [nr-]axSpA) and BE MOBILE 2 (radiographic [r-]axSpA i.e., ankylosing spondylitis).1 One of the most common questions patients ask their healthcare providers is, “how quickly will I feel better after starting this new drug?” Here, we assess the rapidity of response to treatment after a single dose, and subsequent doses of BKZ in patients with axSpA, using data from two phase 3 studies.
Methods: The BE MOBILE 1 (NCT03928704) and 2 (NCT03928743) studies were double-blind, consisting of a 16-week placebo (PBO)-controlled period and a 36-week maintenance period. Patients were randomized to subcutaneous BKZ 160 mg every 4 weeks (Q4W) or PBO for 16 weeks, with all patients receiving BKZ 160 mg Q4W from Week 16 onwards.
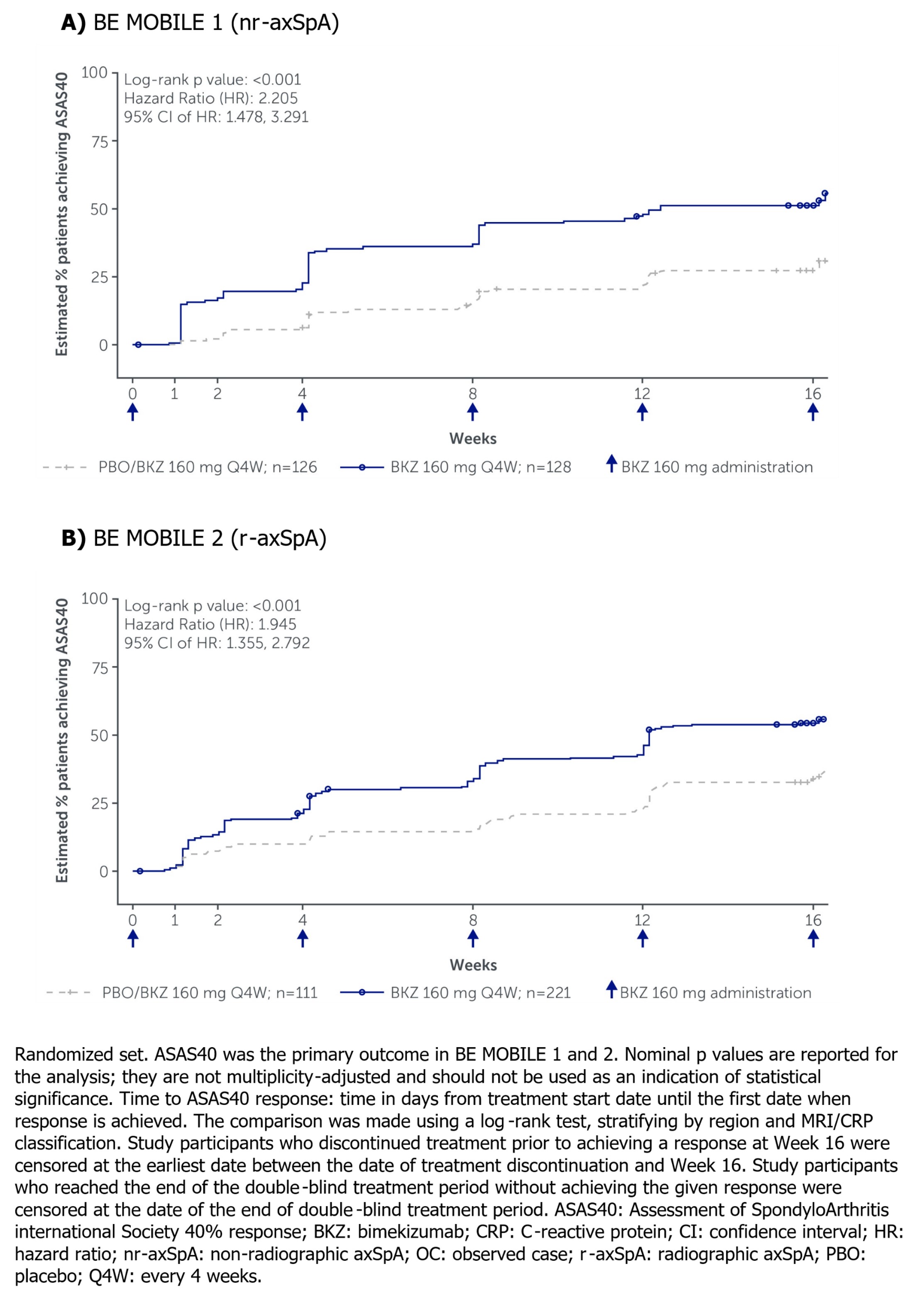
Here we present treatment responses over the first 16 weeks for the BKZ and PBO treatment arms, including Kaplan-Meier analyses of Assessment of SpondyloArthritis international Society 40% (ASAS40) response, using observed case (OC) imputation. Non-responder imputation (NRI) and multiple imputation (MI) were applied for missing binary and continuous outcomes, respectively. Aside from p values reported at Week 16 for the ranked primary (ASAS40) and secondary endpoints of each study, all other p values are nominal.
Results: Of the 254 patients enrolled in BE MOBILE 1 (BKZ: 128; PBO: 126) and 332 in BE MOBILE 2 (BKZ: 221; PBO: 111), 96.1% (244/254) and 97.0% (322/332) completed to Week 16, respectively.
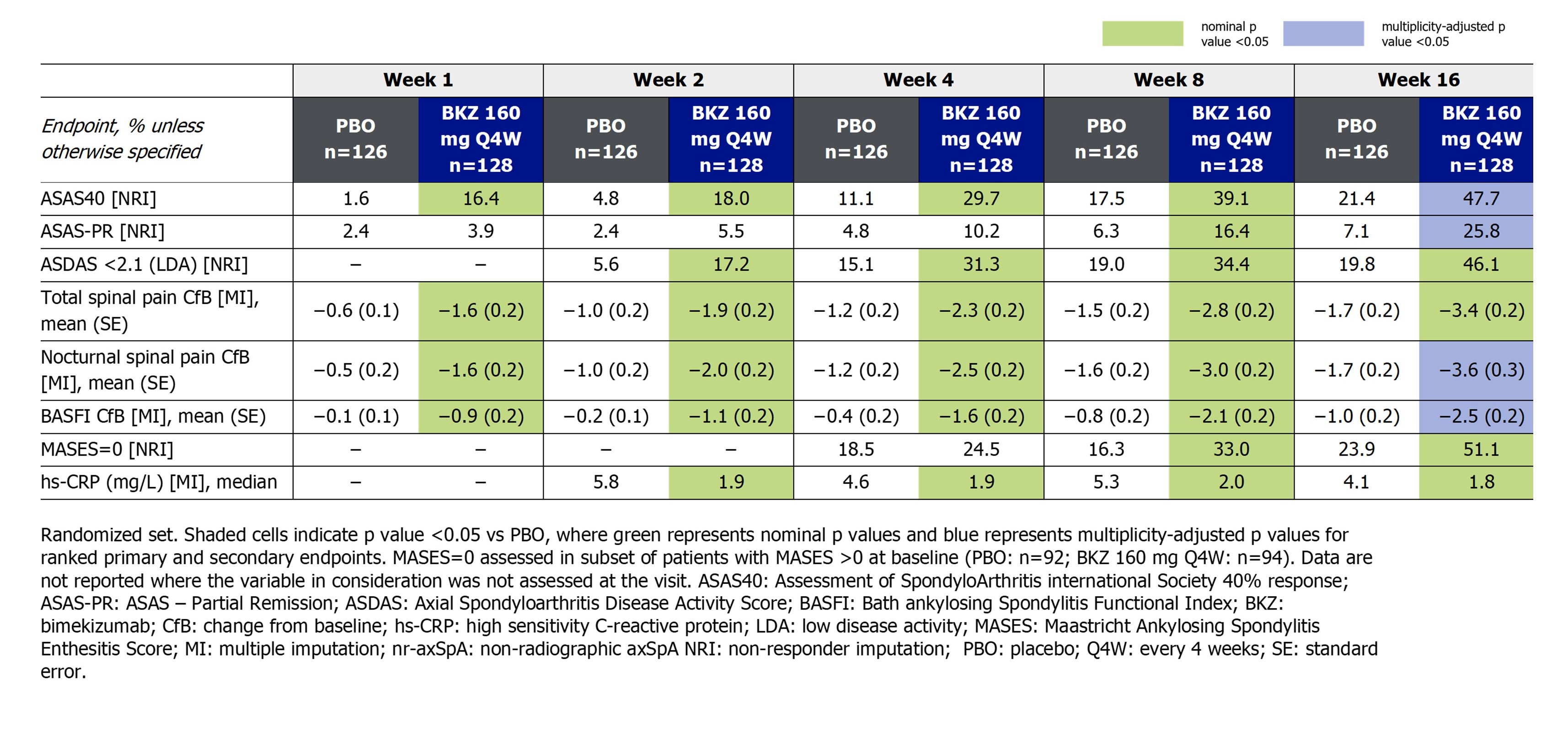
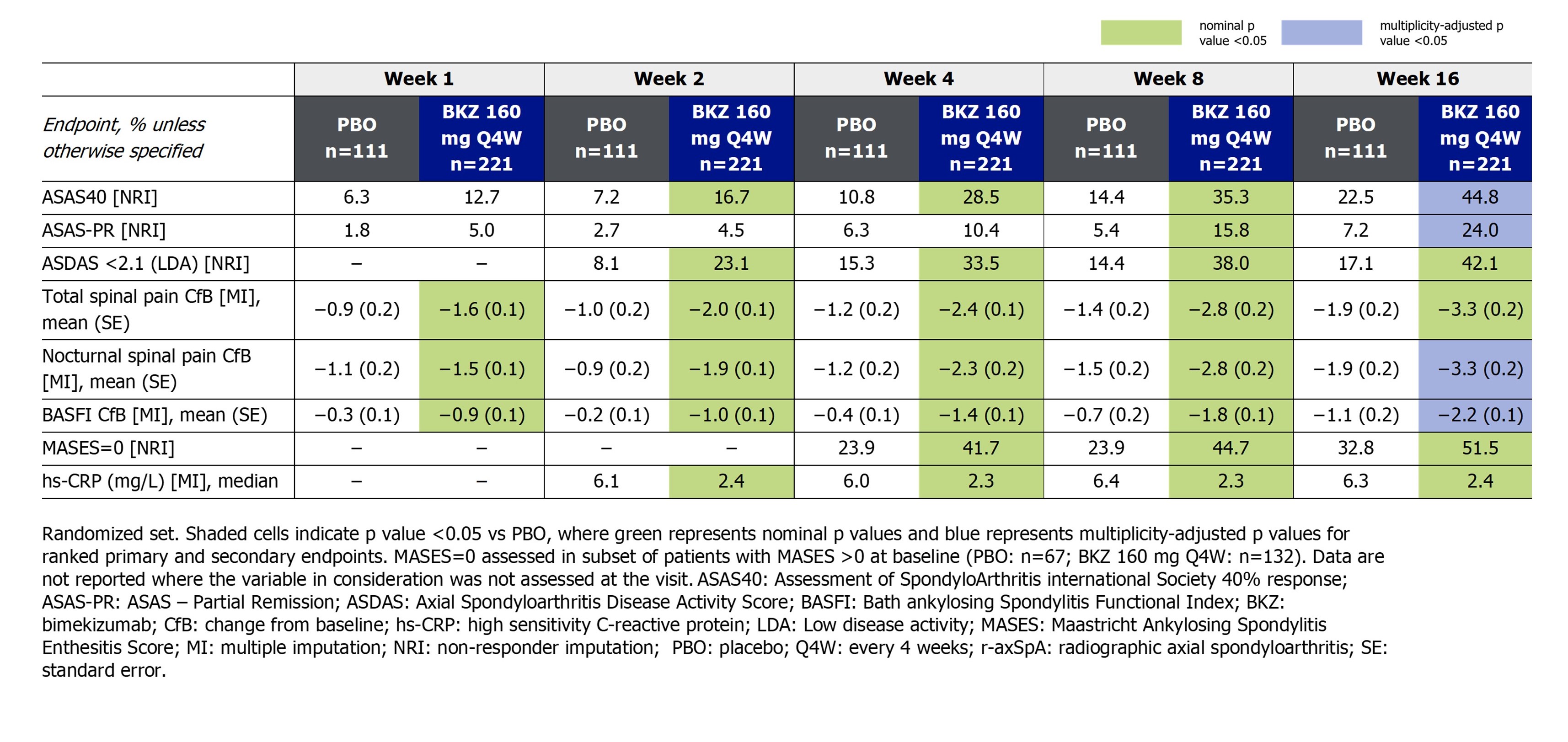
Kaplan-Meier analyses showed early separation between BKZ and PBO for ASAS40 (Figure), with a greater proportion of patients achieving ASAS40 after a single dose of BKZ at baseline, from Week 1 for nr‑axSpA patients (16.4% vs 1.6%; Table 1) and from Week 2 for r-axSpA patients (16.7% vs 7.2%; Table 2). ASAS40 response rates continued to increase to Week 16 in all patients.
For many of the other endpoints, separation between BKZ vs PBO was observed after a single dose of BKZ at baseline, from Week 1 in patients with nr-axSpA and Week 2 in patients with r-axSpA. This included the proportion of patients achieving Axial Spondyloarthritis Disease Activity Score low disease activity (< 2.1). Further outcomes, including improvements in spinal pain and physical function, are reported in Table 1–2.
Conclusion: Patients across the full disease spectrum of axSpA treated with BKZ achieved rapid treatment responses, with early separation from PBO as early as 1–2 weeks after a single dose of BKZ at baseline. These results are of practical importance for counseling patients with axSpA.
References:
1. Baraliakos X. Ann Rheum Dis 2024; 83:199–213.
To cite this abstract in AMA style:
Deodhar A, Nikiphorou E, Danve A, Hall S, Taieb V, Voiniciuc D, Magrey M, Baraliakos X. “How Quickly Will I Feel Better with This New Drug?” – Rapidity of Treatment Response in Patients with Axial Spondyloarthritis Treated with Bimekizumab: Analysis from Two Phase 3 Studies [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/how-quickly-will-i-feel-better-with-this-new-drug-rapidity-of-treatment-response-in-patients-with-axial-spondyloarthritis-treated-with-bimekizumab-analysis-from-two-phase/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/how-quickly-will-i-feel-better-with-this-new-drug-rapidity-of-treatment-response-in-patients-with-axial-spondyloarthritis-treated-with-bimekizumab-analysis-from-two-phase/