Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The existing guidelines for remission induction in lupus nephritis (LN) from both the ACR and the EULAR recommend initial prednisone doses of 0.5-1mg/kg/day. However, recent observational studies reported non-inferior outcomes with significantly lower doses. The aim of this study was to compare the complete renal response rates in LN patients treated initially with £30mg/day or ³40mg/day of prednisone.
Methods: Patients with new-onset LN and standard immunosuppressive treatment with azathioprine or mycophenolate mofetil in standard doses or cyclophosphamide (Euro-lupus protocol) were included and followed for at least 12 months. Subjects were divided into low-to-medium (£30mg/day) and high prednisone groups (³40mg/day) and propensity score-matched based on global and renal disease activity. Complete renal response was defined as proteinuria < 0.5g/day and no worsening in renal function (serum creatinine £120% from baseline). Glucocorticoid-related damage was also assessed.
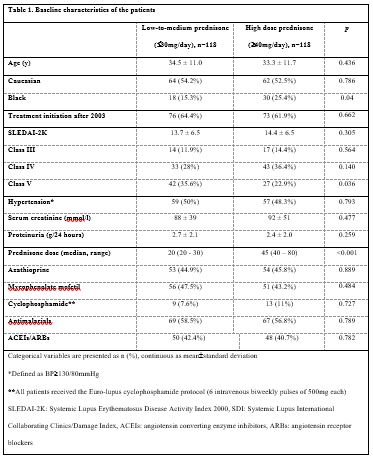
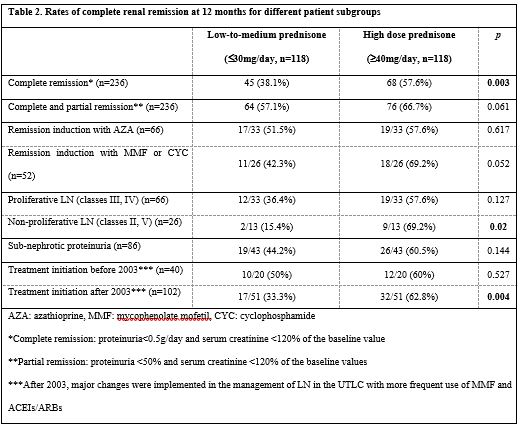
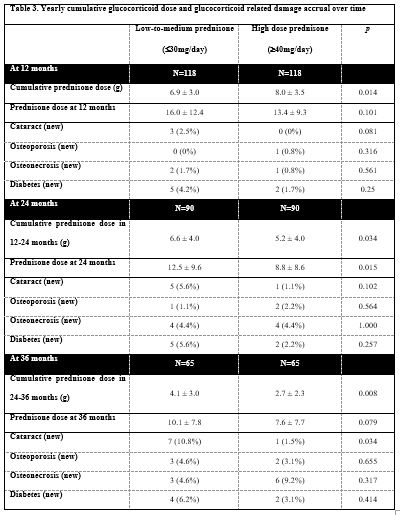
Results: Two hundred and thirty-six patients (118 in each group) were included. Baseline characteristics were well-balanced between groups except Black patients’ predominance in the high prednisone group (25.4% vs. 15.3%, p=0.04) and higher frequency of LN class V in the low dose group (35.6% vs. 22.9%, p=0.036), Table 1. Median prednisone doses were 45mg/day and 20mg/day for the high and low-to-medium dose groups respectively. Complete renal response rates at 12 months in the main groups and certain subgroups of patients are shown in Table 2. Complete remission rates were also higher at two [75% vs. 40.3%, p=0.0002] and three years [67.2% vs. 51.7%, p=0.144] after LN diagnosis. Patients in the high dose group received less cumulative glucocorticoids during the 2nd and 3rd year after LN diagnosis and did not accrue more glucocorticoid-related damage, Table 3.
Conclusion: Higher initial prednisone doses (median 45mg/day) achieved significantly better rates of complete renal response at 12 and 24 months in new-onset LN. Findings were similar in different patient subgroups. These patients received less cumulative glucocorticoids in the 2nd and 3rd year and did not accrue more glucocorticoid-related damage. Our findings suggest that the treatment of LN with initially high doses of prednisone leads to improved rates of renal response that, in turn, allows for faster glucocorticoid tapering compared to patients who were treated with lower doses.
To cite this abstract in AMA style:
Tselios K, Gladman D, Al-Sheikh H, Su J, Urowitz M. How Much Prednisone Is Enough for Remission Induction in Lupus Nephritis? A Propensity Score Matched Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/how-much-prednisone-is-enough-for-remission-induction-in-lupus-nephritis-a-propensity-score-matched-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/how-much-prednisone-is-enough-for-remission-induction-in-lupus-nephritis-a-propensity-score-matched-analysis/