Session Information
Date: Saturday, November 16, 2024
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: JAK-inhibitors (JAKi) have demonstrated a rapid onset of action; however, it is unclear how they compare to other targeted therapies in rheumatoid arthritis (RA). The aim of this study is to evaluate and compare the short-term (3-month) effectiveness of JAKi and bDMARDs in RA patients in a real-world population from an international collaboration of registers (the “JAK-pot” collaboration).
Methods: Patients with RA initiating either JAKi, TNF-inhibitors (TNFi), IL-6 inhibitors (IL-6i) or abatacept (ABA) during the period since JAK-inhibitors became available in each participating country (14 registers) were included. Discontinuation rates were evaluated using survival analysis. Effectiveness was assessed and compared through CDAI, function (HAQ) and pain (0-100 scale) trajectories using polynomial mixed models. Analyses were adjusted for age, gender, seropositivity, disease duration, number of previous b/tsDMARD, and concomitant treatment (csDMARD and glucocorticoid use).
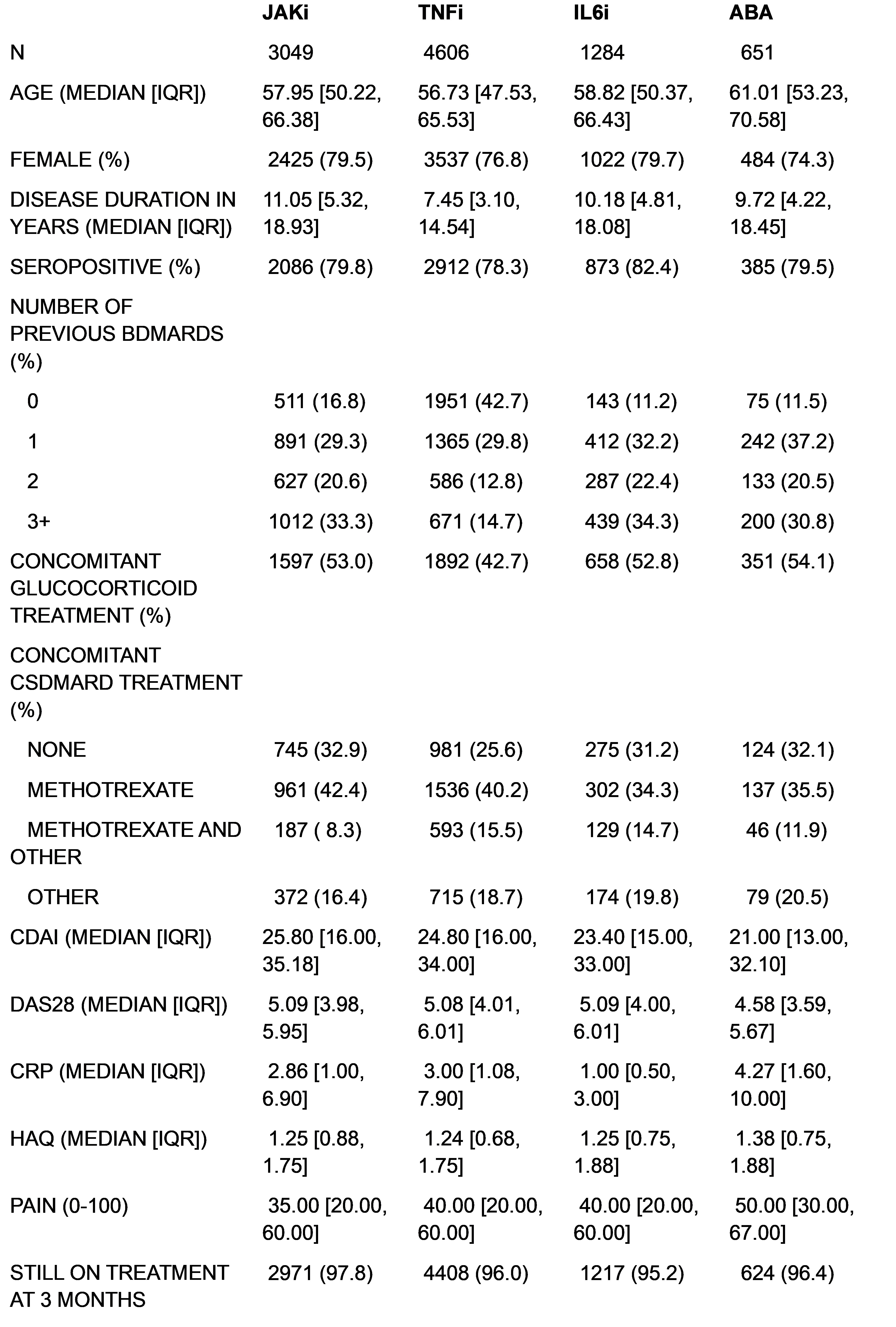
Results: A total of 9590 patients were included (3049 JAKi, 4606 TNFi, 1284 IL-6 I and 651 ABA, Table 1). Patients receiving TNFi were younger, with shorter disease duration, less frequent previous b/tsDMARDs treatment, and less concomitant glucocorticoid or csDMARD than the other groups. Patients receiving ABA were older, with lower disease activity, though CRP was slightly higher than in the other groups. Patients receiving JAKi had the longest disease duration.
Treatment discontinuations were rare in the first 3 months and remained below 10% by 6 months, allowing us to investigate disease activity trajectories, function, and pain.
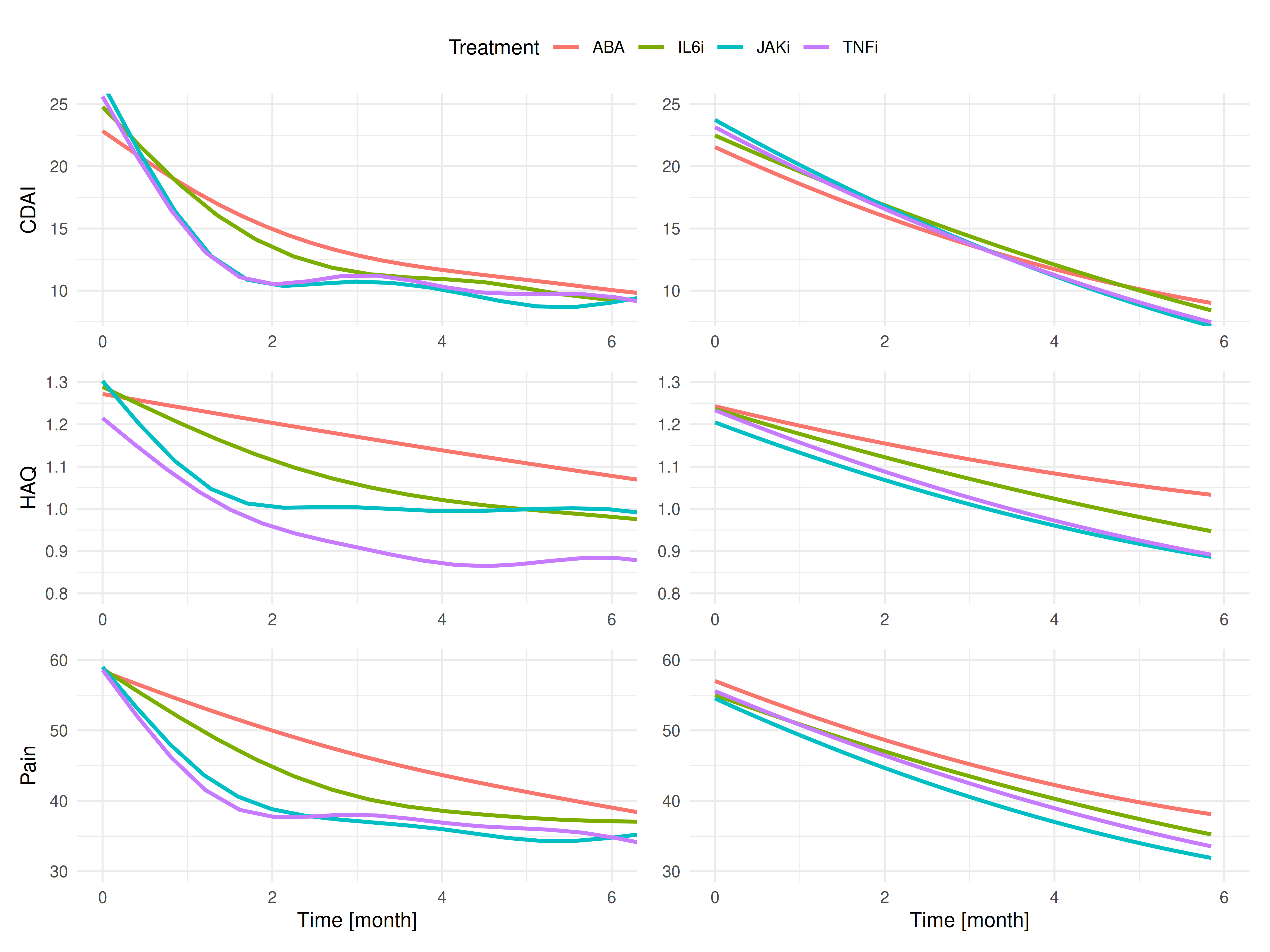
In crude analysis, CDAI improved quickly in the first 2 months, then decreased at a slower rate (Figure 1, top left, ps< 0.001). The decrease was particularly sharp for JAKi, and TNFi, compared to the other treatments (ps< 0.001). For function (Figure 1, middle left), trajectories also improved rapidly in the first months (ps< 0.001) and then stabilized (ps< 0.001), with a significantly slower improvement among patients receiving ABA compared to other targeted therapies. Results were very similar for pain (Figure 1, lower left). After adjustment, the rate of CDAI, HAQ and pain improvement was still faster with JAKi and TNFi, compared to the other bDMARDs (Figure 1, right panels).
Conclusion: The short-term effectiveness of JAKi, TNFi, IL6i and ABA differed, with JAKi and TNFi acting faster on disease activity, function and pain in the first months compared to IL6i and ABA.
To cite this abstract in AMA style:
Courvoisier D, Mrad Y, Mongin D, Aymon R, Caporali R, Choquette D, Codreanu C, Coupal L, Huschek D, Hyrich K, Iannone F, Kvien T, Mustak M, Nordstrom D, Trokovic N, Otero-Varela L, Pavelka K, Pombo Suarez M, Provan S, Rodrigues A, Rotar Z, Sidiropoulos P, Strangfeld A, Závada J, Zhao S, Finckh A, Lauper K. How Fast Do JAK-inhibitors, TNF-inhibitors, Abatacept and IL-6 Inhibitors Act in Rheumatoid Arthritis? An International Collaboration of Registers of Rheumatoid Arthritis Patients (the “JAK-pot” Study) [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/how-fast-do-jak-inhibitors-tnf-inhibitors-abatacept-and-il-6-inhibitors-act-in-rheumatoid-arthritis-an-international-collaboration-of-registers-of-rheumatoid-arthritis-patients-the-jak-pot-stu/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/how-fast-do-jak-inhibitors-tnf-inhibitors-abatacept-and-il-6-inhibitors-act-in-rheumatoid-arthritis-an-international-collaboration-of-registers-of-rheumatoid-arthritis-patients-the-jak-pot-stu/