Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Current therapeutic management of active Class III or IV proliferative LN relies on the use of maintenance therapy following induction. The optimal method for determining the length of maintenance therapy remains a challenge. Most clinicians use a combination of clinical parameters and length of treatment. Unfortunately, there can be a disassociation between clinical parameters and renal biopsies, the gold standard for assessing disease activity.1 Here we present the findings of repeat renal biopsies and a post hoc analysis of their correlation with clinical outcomes from a single site in ALLURE (NCT01714817), a randomized, double-blind Phase III study comparing abatacept (ABA) with placebo (PBO) on background MMF and CS in patients with active Class III or IV LN.
Methods: We evaluated 20 patients from a single site who underwent repeat kidney biopsy (Bx) as part of standard practice. The first Bx was for diagnostic purpose and the second Bx was performed after study discontinuation for clinical decision making. Bx were processed using standard techniques and read, classified using International Society of Nephrology/Renal Pathology Society 2003 Classification and scored using the National Institute of Health activity and chronicity indices (AI/CI) by a single pathologist, blinded to clinical data.2 Renal pathology findings were correlated with clinical outcomes collected during study participation.
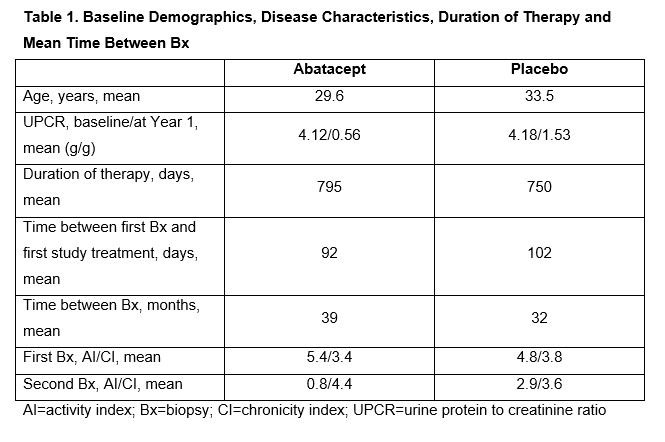
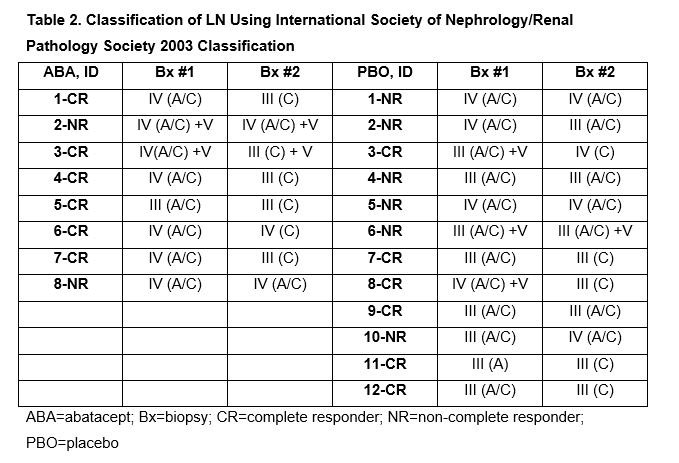
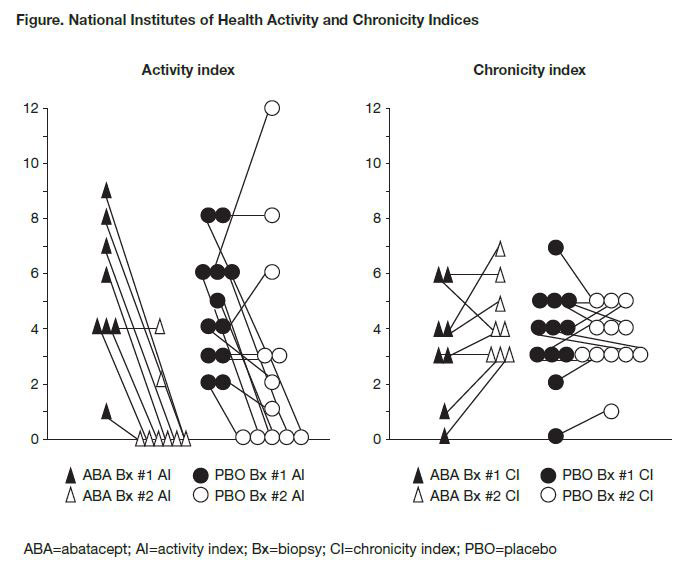
Results: 20/25 patients randomized at the site had paired pre- and post-treatment Bx to be analyzed (ABA n=8; PBO n=12). Demographics and disease characteristics at baseline, duration of therapy and mean time between Bx were comparable (Table 1). 4 patients discontinued treatment, all due to lack of efficacy (ABA n=1; PBO n=3). Most patients achieved a urine protein to creatinine ratio (UPCR) ≤0.5 at least once during therapy (ABA 6/8; PBO 8/12) but mean UPCR at Year 1 was lower in the ABA arm (ABA 0.56; PBO 1.53). While the groups had comparable AI and CI scores at baseline, the ABA group had a lower mean score at the 2nd Bx (0.8 vs PBO 2.9; Table 1). 6/8 of the ABA group had an AI of 0 on repeat Bx vs 5/12 in PBO group (Fig. 1). 2 PBO patients had UPCR ≤0.5 but had some activity on 2nd Bx; 2 ABA patients without activity on 2nd Bx had UPCR >0.5 (0.6 and 0.8). After an average of >2 years of treatment, most responders were classified as III (C) on a repeat Bx (Table 2). Overall safety was comparable in both groups; 2 ABA patients had serious infections (pneumonia and gastroenteritis) that did not lead to discontinuation.
Conclusion: Repeat kidney Bx in patients with active LN after prolonged therapy can reveal discrepancies between clinical and histological responses. Most patients treated with abatacept showed lack of activity even with mild, residual proteinuria. Better methodologies are needed to detect renal activity and improve clinical decision making in patients with LN.
References
- Roseman DA, et al. Nephrol Dial Transplant 2017;32:1344–50.
- Austin HA 3rd, et al. Am J Med 1983;75:382–91.
Writing support: Carol Keys, Caudex; funding: Bristol-Myers Squibb
To cite this abstract in AMA style:
Malvar A, Alberton V, Recalde C, Gao S, Maldonado M. Histologic Findings from Paired Renal Biopsies and Clinical Outcomes: Results from a Single Site in the Phase III Study of Abatacept in Patients with Proliferative LN [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/histologic-findings-from-paired-renal-biopsies-and-clinical-outcomes-results-from-a-single-site-in-the-phase-iii-study-of-abatacept-in-patients-with-proliferative-ln/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/histologic-findings-from-paired-renal-biopsies-and-clinical-outcomes-results-from-a-single-site-in-the-phase-iii-study-of-abatacept-in-patients-with-proliferative-ln/