Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Secukinumab provided sustained improvement in the signs and symptoms of ankylosing spondylitis (AS) over 2 years in the MEASURE 2 study (NCT01649375).1,2 Here we report the efficacy and safety of secukinumab over 3 years from the study.
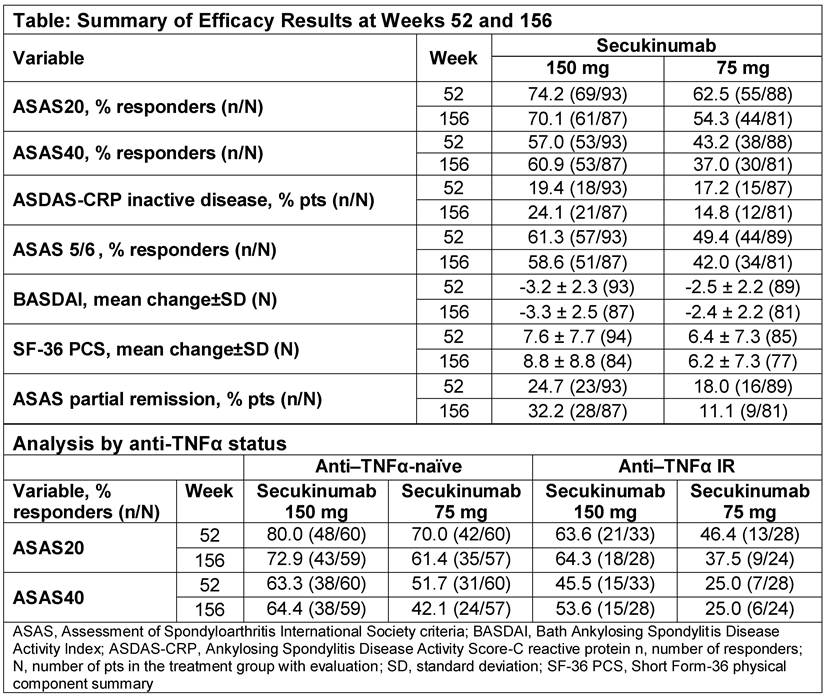
Methods: 219 patients (pts) with active AS were randomized to subcutaneous secukinumab 150 mg (72 pts), 75 mg (73 pts) or placebo (PBO, 74 pts) at baseline. At Week (Wk) 16, PBO treated pts were re-randomized 1:1 to secukinumab 150 or 75 mg, irrespective of clinical response. Pts initially randomized to secukinumab and those who switched from PBO to secukinumab at Wk 16 were included in the analysis (secukinumab 150 mg, N = 106 and secukinumab 75 mg, N = 105). Outcome measures at Wk 156 included ASAS20 and 40, ASDAS-CRP inactive disease, ASAS5/6, BASDAI, SF-36 PCS and ASAS partial remission. Analyses stratified by anti-TNFα status (anti–TNFα-naïve and anti-TNFα inadequate response [IR]) were pre-specified. Data are reported as observed. Safety analyses included all pts who received ≥1 dose of secukinumab.
Results: At Wk 156, the completion rate for secukinumab 150 mg was 81.1% (86/106), compared to 72.4% (76/105) for 75 mg. The higher discontinuation rate for 75 mg was in part due to lack of efficacy or patient/guardian decision. Efficacy observed across endpoints from Wks 52 to 156 is summarized in the Table. Higher responses were observed in the 150 mg group. Improvements in ASAS20 and ASAS40 responses were sustained in anti–TNFα-naïve and anti–TNFα-IR pts (Table). Over the entire study period, the mean exposure [± SD] to secukinumab was 914.3 ± 315.5 days. The safety and tolerability profile of secukinumab was consistent with previous reports; nasopharyngitis, upper respiratory tract infection, diarrhoea and bronchitis were the most frequently reported adverse events (AEs). The exposure-adjusted incidence rates with secukinumab for AEs of interest were serious infections/infestations (1.5), Crohn’s disease (0.6), malignant/unspecified tumours (0.6) and major adverse cardiovascular events (0.6) per 100 pt-years.
Conclusion: Secukinumab 150 mg provided sustained response in the signs and symptoms along with physical function through 3 years in pts with AS, with over 80% retention rate. The safety profile remained favourable and was consistent with previous reports.1,2
Reference: 1. Baeten et al. N Engl J Med 2015;373:2534‒48; 2. Marzo-Ortega H, et al. Arthritis Care Res (Hoboken) 2017;doi: 10.1002/acr.23233
To cite this abstract in AMA style:
Kivitz AJ, Marzo-Ortega H, Legerton C, Sieper J, Blanco R, Cohen M, Delicha EM, Rohrer S, Richards H. High Retention Rate and Sustained Responses with Secukinumab 150mg in Patients with Active Ankylosing Spondylitis: 3-Year Results from a Phase 3 Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/high-retention-rate-and-sustained-responses-with-secukinumab-150mg-in-patients-with-active-ankylosing-spondylitis-3-year-results-from-a-phase-3-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-retention-rate-and-sustained-responses-with-secukinumab-150mg-in-patients-with-active-ankylosing-spondylitis-3-year-results-from-a-phase-3-study/