Session Information
Date: Saturday, November 7, 2020
Title: RA – Treatments Poster II: Comparative Effectiveness, Biosimilars, Adherence & the Real World
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Recent developments of targeted treatments such as targeted synthetic DMARDs (tsDMARDs) increase the chances of a sustained low disease activity (LDA) or remission state for patients suffering rheumatoid arthritis (RA). tsDMARDs such as baricitinib, an oral inhibitor of the Janus Kinases (JAK1/JAK2) was recently approved for the treatment of RA with an inadequate response to conventional (cDMARD) and biological (bDMARD) therapy.
(1, 2).
Aim of this study is to analyze the effect of baricitinb on disease activity (DAS28, LDA) in patients with RA in real life, to analyze drug persistance and associate these effects with various baseline characteristics.
Methods: All RA patients were seen in our outpatient clinic. If a patient was switched to a baricitinib due to medical reasons, these patients were included in our prospective, observational study which started in April 2017. Clinical scores (SJC/TJC 76/78), composite scores (DAS28), PROs (HAQ-DI; RAID; FACIT), safety parameters (not reported in this abstract) as well as laboratory biomarkers were collected at each visit every three months. Linear mixed effects models for repeated measurements were used to analyze the time course of disease activity, patient reported outcomes and laboratory results. We estimated the probabilities of continued baricitinib treatment and the probabilities of LDA and remission by DAS-28 as well as Boolean remission up to one year using survival analysis and explored their association with disease characteristics using multivariable Cox regression. All patients gave informed consent. The study is approved by the local ethics.
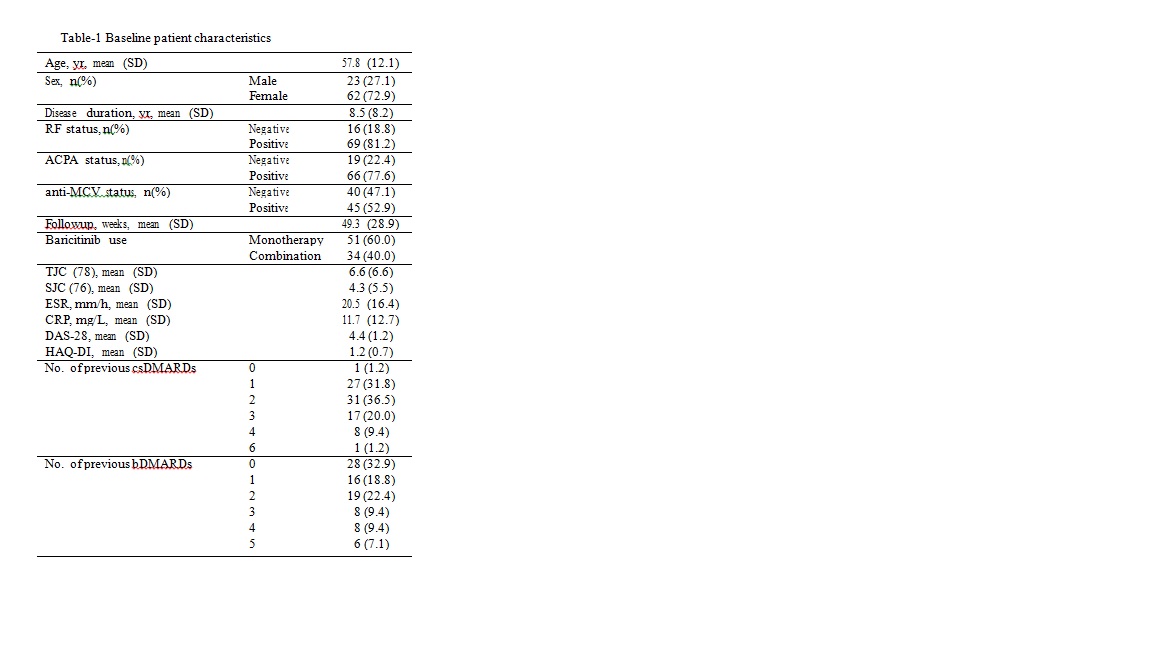
Results: 95 patients were included and 85 analyzed with available follow-up data until November 2019. Demographics are shown in table 1. Mean follow-up duration after starting baricitinib was 49.3 (28.9) weeks. 51 patients (60%) were on monotherapy. Baricitinib survival (95%CI) was 82% (73% to 91%) at one year. Cumulative number (%probability, 95%CI) of patients that attained DAS-28 LDA at least once up to one year was 67 (92%, 80% to 97%) and the number of patients attaining DAS-28 and Boolean remission were 31 (50%, 34% to 61%) and 12(20%, 9% to 30%) respectively. Median time to DAS-28 LDA was 16 weeks (Figure-1). Cox regression analyses did not show any sufficiently precise association of remission or LDA with age, gender, seropositivity, disease duration, concomitant DMARD use and number of previous bDMARDs. Increasing number of previous bDMARDs was associated with poor baricitinib survival (HR=1.5, 95%CI 1.1 to 2.2) while this association was not robust to adjustment for baseline disease activity. Favorable changes were observed in tender and swollen joint counts, pain-VAS, patient and physician disease assessment scores, RAID, FACIT and the acute phase Response.
Conclusion: In this prospective observational study, we observed high rates of LDA and DAS-28 remission and significant improvements in disease activity and patient reported outcome measurements over time.
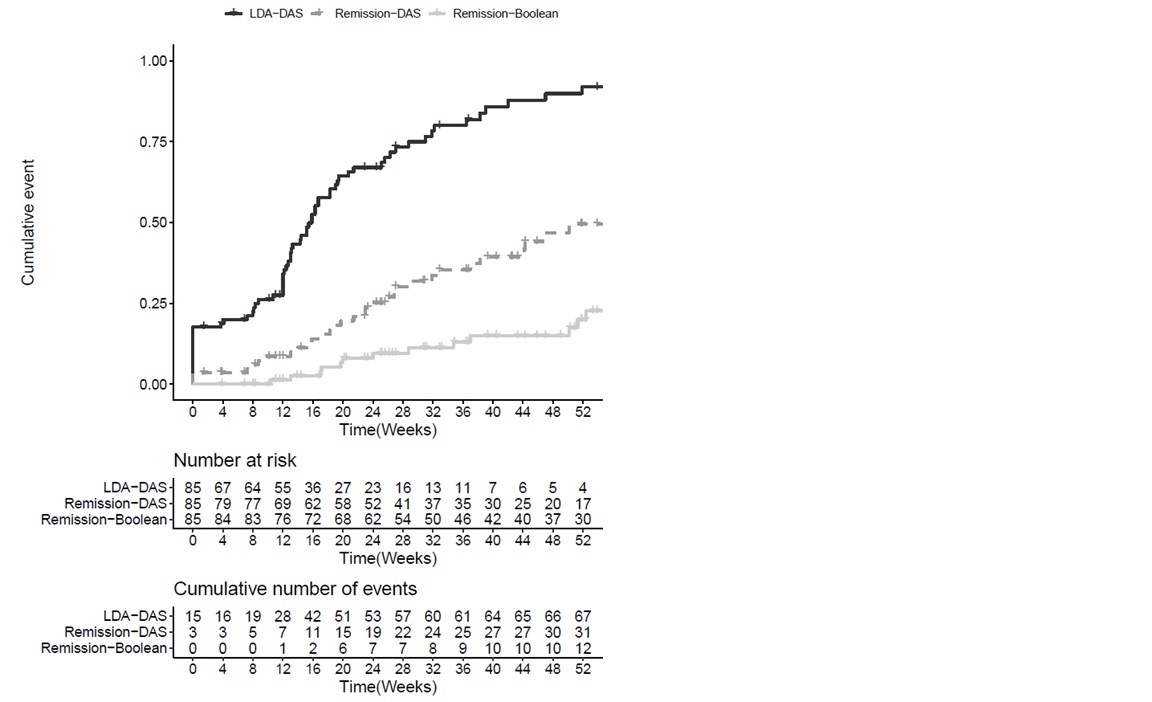 Figure-1 Cumulative probability of low disease activity or remission under treatment with baricitinib.
Figure-1 Cumulative probability of low disease activity or remission under treatment with baricitinib.
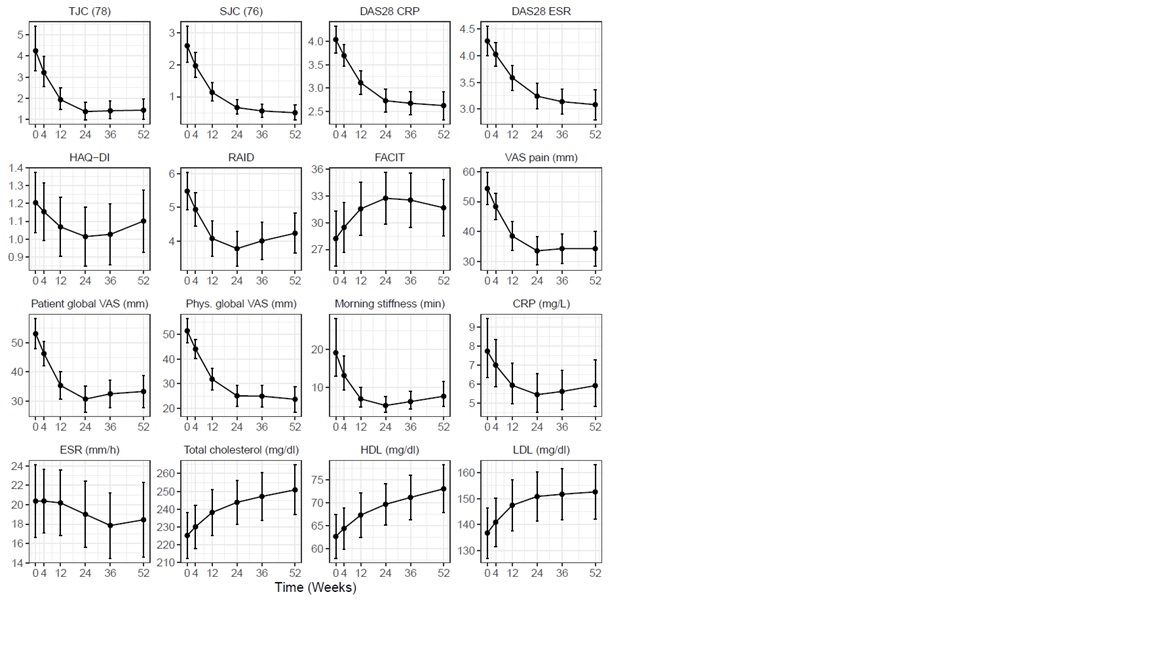 Figure-2 Course of disease activity measures, patient reported outcome measures, acute phase response and cholesterol levels over treatment course in baricitinib users.
Figure-2 Course of disease activity measures, patient reported outcome measures, acute phase response and cholesterol levels over treatment course in baricitinib users.
To cite this abstract in AMA style:
Bayat S, Tascilar K, Kleyer A, Simon D, Hueber A, Schett G. High Remission Rates in RA – Real Life Data from Bariticinib [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/high-remission-rates-in-ra-real-life-data-from-bariticinib/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-remission-rates-in-ra-real-life-data-from-bariticinib/