Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The 2013 American Academy of Orthopaedic Surgeons (AAOS) clinical practice guideline (CPG) on the treatment of knee OA made a strong recommendation not in favor of the use of intraarticular hyaluronic acid (IAHA) because, though statistically significant, improvement in pain did not meet the threshold for a minimal clinically important difference (MCID). The AAOS CPG, and publications since, suggest there may be clinically important differences in the effectiveness of high vs. low molecular weight (MW) IAHA. We aimed to update the evidence-base since the guideline publication and use a network meta-analysis (NMA) to incorporate both direct and indirect evidence on this issue.
Methods: MEDLINE, Embase and CENTRAL were systematically searched to identify RCTs evaluating non-surgical interventions for people with knee OA. Trials were selected based on the AAOS CPG eligibility criteria. Effect sizes from each trial were computed using a pain measure hierarchy and longest follow up. The mean difference between intervention and comparator was converted to the standardized mean difference (SMD) before incorporating into the NMA using a random-effects Bayesian framework. To assess the impact of MW, high MW (HMW; defined as at least 6000 kDa) and low MW (LMW; < 750 kDa) nodes were created. Studies of IAHA which fell outside this definition were excluded. Sensitivity analyses were used to examine the robustness of the findings.
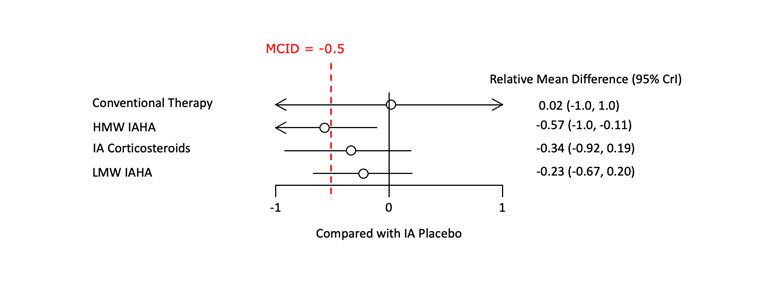
Results: The base-case analysis included a total of 14 RCTs (N=2,793), creating a network with 5 therapeutic nodes (Figure 1). Trials on NSAIDs, acetaminophen, and oral opioids were excluded as they lacked network connectivity. The results indicated that compared to IA placebo, HMW IAHA had a statistically significant effect on pain, which was “possibly clinically significant” according to the AAOS definition (SMD -0.57 [95% Credible Interval: -1.0, -0.11]) (Figure 2). In contrast, LMW IAHA resulted in a small, non-statistically significant improvement in pain relative to IA placebo (-0.23 [-0.67, 0.20]). The Bayesian model showed that HMW IAHA had the highest probability (71%) of ranking 1st with regard to improvement in pain score. Back-transforming the SMD to the WOMAC 0-100 pain scale indicated a 14.65 [13.93, 15.62] point improvement over IA placebo, substantially better than the AAOS minimum clinically important improvement threshold of 8.3. Sensitivity analyses confirmed these findings were robust.
Conclusion: Based on direct and indirect evidence, stratified data of HMW IAHA (≥6000 kDa) revealed a pooled effect in pain relief which exceeded even the most conservative threshold for a MCID. Meaning, on average, the improvement can be subjectively perceived by the affected persons under therapy. In contrast, the pooled effect of LMW IAHA (< 750 kDa) is neither statistically significant nor clinically relevant. Amalgamation of data of LMW and HMW IAHA may have blurred the benefits of IAHA, which have led to negative therapy recommendations in the past. Differentiation according to the molecular weight offers refined insight into the IAHA treatment. The sub class of HMW IAHA’s should be further evaluated in new focused RCTs.
To cite this abstract in AMA style:
Hummer C, Angst F, Schemitsch E, Whittington C, Manitt C, Ngai W. High Molecular Weight Intraarticular Hyaluronic Acid for the Treatment of Knee Osteoarthritis: A Network Meta-Analysis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/high-molecular-weight-intraarticular-hyaluronic-acid-for-the-treatment-of-knee-osteoarthritis-a-network-meta-analysis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-molecular-weight-intraarticular-hyaluronic-acid-for-the-treatment-of-knee-osteoarthritis-a-network-meta-analysis/