Session Information
Date: Sunday, November 10, 2019
Title: RA – Diagnosis, Manifestations, & Outcomes Poster I: Risk Factors, Predictors, & Prognosis
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Depression in rheumatoid arthritis (RA) patients may be pre-existent, amplified, or may develop after the onset of RA1. The Patient Health Questionnaire (PHQ9) is a widely-used tool to screen for depression in primary care2. A multidimensional health assessment questionnaire (MDHAQ)3 includes two screening queries for depression. MDHAQ also includes routine assessment patient index data (RAPID3), an index which is correlated significantly with disease activity score (DAS28) and clinical disease activity index (CDAI), and also is informative in all rheumatic diseases in which it has been studied4. We investigated possible associations of PHQ9 scores for depression with MDHAQ demographic, clinical, psychological and laboratory measures.
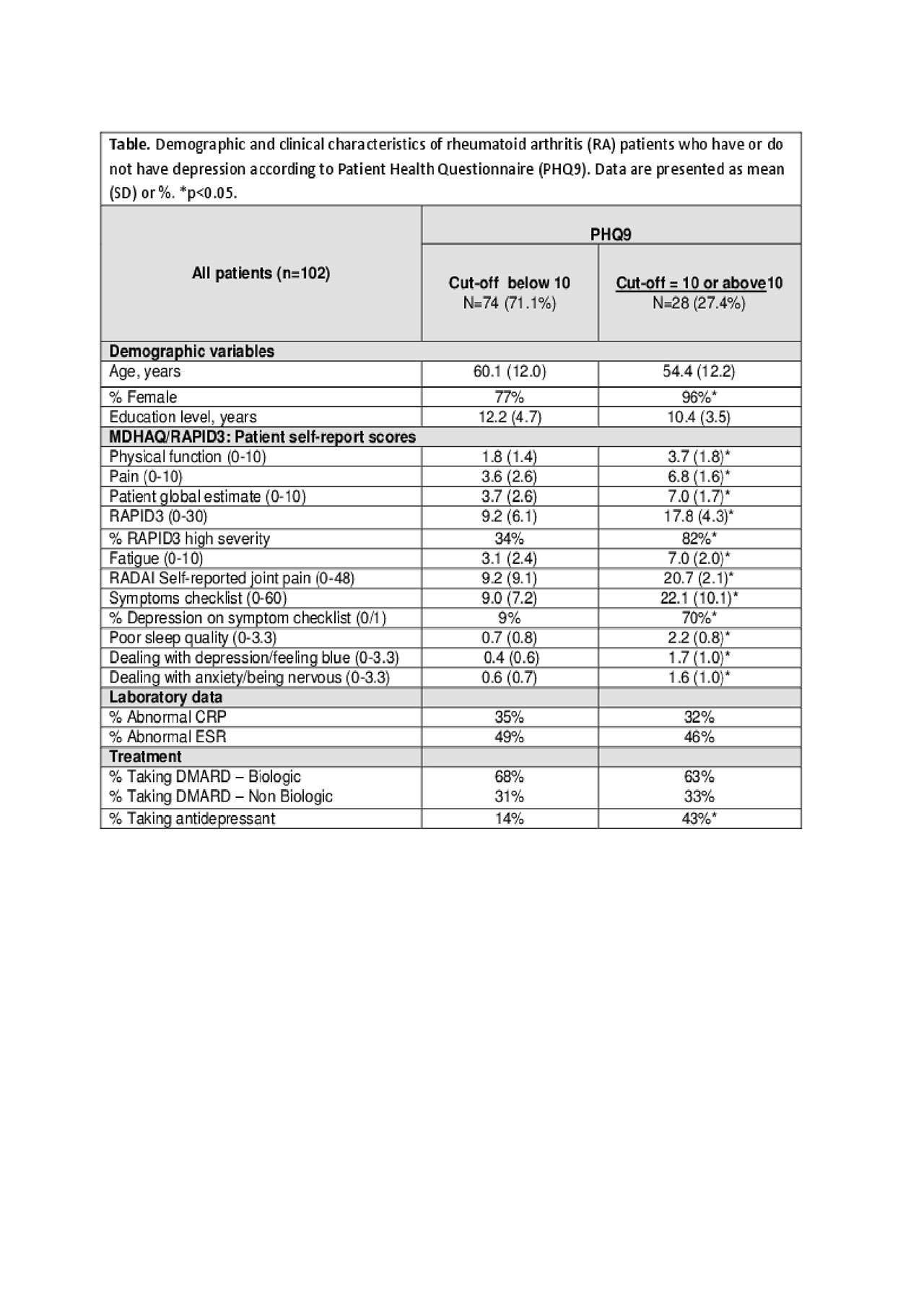
Methods: Consecutive patients with RA, who met EULAR/ACR 2010 criteria, completed both an MDHAQ and a PHQ9 as part of a routine rheumatology visit between November 2018 and February 2019. The 2-page MDHAQ includes 0-10 scores for physical function (FN), and visual analogue scales (VAS) for pain (PN) and patient global estimate (PATGL), compiled into 0-30 RAPID3 (≤3 =remission, 3.1-6=low, 6.1-12=moderate, and >12=high severity), 0-10 fatigue (FT) VAS, 0-48 RADAI self-report painful joint count, and 0-60 symptom checklist, which includes depression. The MDHAQ also includes 3 psychological queries in the patient-friendly HAQ format for depression, anxiety, and poor sleep quality, as well as demographic data. PHQ9 is a 9-item self-report screening tool for depression in primary care (range 0-27); PHQ9 scores ≥ 10 indicate positive screening for depression. MDHAQ demographic, self-report clinical, psychological and laboratory variables were compared according to PHQ9 depression group, ≥10 vs < 10.
Results: 102 RA patients were included in the analysis: 82.3% were females, mean age was 58.8 years and mean formal educational level of 11.7 years. 28 patients (27.4%) had PHQ9 ≥ 10, positive for depression (Table). PHQ9 ≥ 10 patients did not differ significantly from PHQ9 < 10 patients in age and educational level, while 96% of PHQ9 ≥ 10 patients were female (p=0.02). Patients who reported PHQ9 ≥ 10 had significantly higher scores vs those with PHQ9 < 10 for physical function, pain, PATGL, and RAPID3 (Table). High RAPID3 severity ( >12) was seen in 82% of PHQ9 ≥ 10 patients versus 34% of PHQ9 < 10 patients. Patients with PHQ9 ≥ 10 also had significantly higher scores on the two MDHAQ depression queries vs those with PHQ9 < 10 (Table). No differences in the two groups were seen in acute phase reactants ESR and CRP or in treatment with DMARDs. 43% of patients with a PHQ9 ≥ 10 were being treated for depression vs 14% of PHQ9 < 10 patients (Table).
Conclusion: RA patients screening positively for depression according to PHQ9 have high scores for all MDHAQ, including depression scale as well as physical function, pain, RAPID3, fatigue, and others, indicating a high self-report disease burden.
References: 1. J Clin Rheumatol. 2017;23:425-434. 2. J Gen Intern Med. 2001;16:606-13. 3. Rheum Dis Clin North Am. 2009;35:787-98. 4. Bull Hosp Jt Dis. 2017;75:93-100.
To cite this abstract in AMA style:
Morlà R, Riad M, Castellanos-Moreira R, Ruiz-Esquide V, Sanmarti R, Gomez-Puerta J, Pincus T, Castrejon I. High MDHAQ/RAPID3 (Multidimensional Health Assessment Questionnaire/Routine Assessment of Patient Index Data) Scores in RA Patients Who Have Depression According to a Screening Questionnaire [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/high-mdhaq-rapid3-multidimensional-health-assessment-questionnaire-routine-assessment-of-patient-index-data-scores-in-ra-patients-who-have-depression-according-to-a-screening-questionnaire/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-mdhaq-rapid3-multidimensional-health-assessment-questionnaire-routine-assessment-of-patient-index-data-scores-in-ra-patients-who-have-depression-according-to-a-screening-questionnaire/