Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with immune-mediated inflammatory disorders (IMIDs) have an inherently heightened susceptibility to infection and may be considered high risk for COVID-19, yet IMID populations were initially excluded from vaccine efficacy studies. We have previously shown that: (1) at 3 months post vaccination, IMID patients on background methotrexate (MTX) were less likely to achieve an adequate humoral response compared to healthy controls and IMID patients on other immunomodulatory medications and (2) at 6 months, those on MTX, tumor necrosis factor inhibitors (TNFis), or combination MTX-TNFi therapy were less likely to maintain an adequate humoral response. The objective of this study was to extend the characterization of the humoral immune response to mRNA COVID-19 vaccines in patients with IMID on immunomodulatory treatment to 12 months post vaccination.
Methods: Established patients with IMID and healthy controls at NYU receiving COVID-19 vaccination were assessed for humoral immune response at 4/5 weeks, 3 months, 6 months, and 12 months post first vaccine dose. Humoral response was assessed by testing IgG antibodies against the S1 domain of the spike protein of SARS-CoV-2. In line with our prior studies, adequate response was defined as greater than 5,000 RU/ml (although no titer is known to indicate immunity). Patient characteristics were summarized using means, standard deviations, and percentages as appropriate. Chi squared tests of independence and Fisher’s exact tests were used for categorical variables between groups; Kruskal-Wallis tests for continuous variables as appropriate.
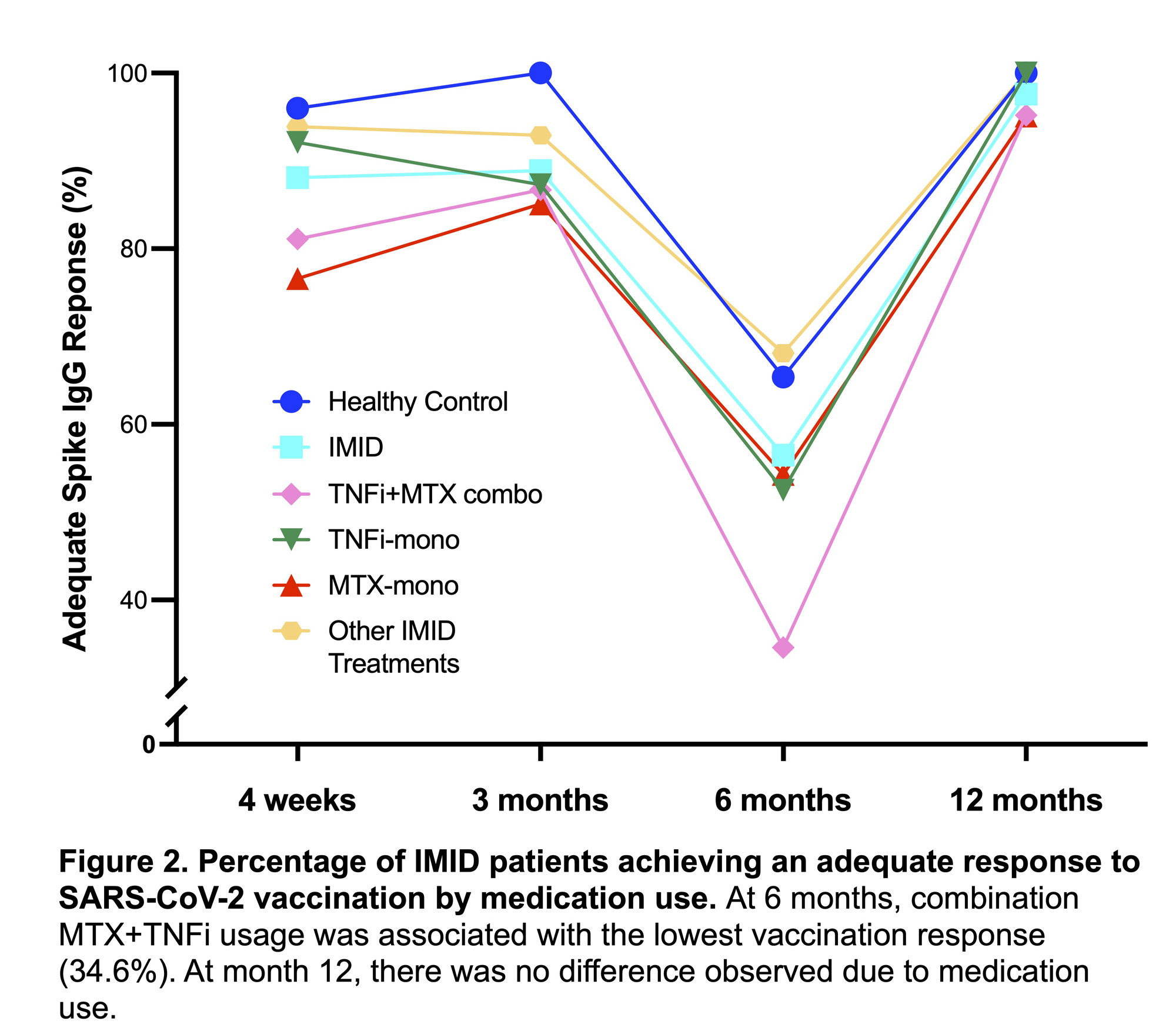
Results: By 12 months, 23/23 of the healthy controls and 79/83 of the participants with IMIDs received a supplemental dose of the COVID19 booster (Table 1). Participants with IMID were an average of 130 days since the booster dose at the time of phlebotomy (compared to 78 days in the healthy controls). All healthy controls and 81/83 IMID participants achieved an adequate humoral response and there was no difference in antibody titer levels (Figure 1). Antibody titer levels did not correlate with time since booster dose (r= -0.18, p=0.12). Of the 17 patients who had an initial non-response at week 4, all but one demonstrated an adequate response at 12 months. Additionally, there was no difference in response rate or antibody titer level by medication use (Figure 2).
Conclusion: Here, we demonstrate the immunogenicity of a COVID-19 booster dose, regardless of medication use and even in patients who did not initially mount a response. While there is no antibody titer that correlated with clinical efficacy, these findings highlight the importance of a supplemental dose in patients with IMID. Larger scale studies and those looking at the bivalent booster shot are needed to confirm and better understand these findings.
To cite this abstract in AMA style:
Haberman R, Blank R, Rackoff P, Solomon G, Adhikari S, Azar N, Herati R, Rosenthal P, Izmirly P, Samuels J, Golden B, Reddy S, Mulligan M, Scher J. High Immunogenicity of mRNA Covid-19 Vaccine Booster in Immune Mediated Inflammatory Disease [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/high-immunogenicity-of-mrna-covid-19-vaccine-booster-in-immune-mediated-inflammatory-disease/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/high-immunogenicity-of-mrna-covid-19-vaccine-booster-in-immune-mediated-inflammatory-disease/